The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue
- PMID: 14557547
- PMCID: PMC218773
- DOI: 10.1073/pnas.1635213100
The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue
Abstract
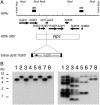
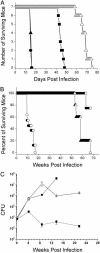
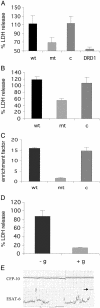
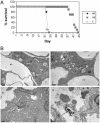
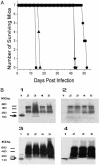
Tuberculosis remains a leading cause of death worldwide, despite the availability of effective chemotherapy and a vaccine. Bacillus Calmette-Guérin (BCG), the tuberculosis vaccine, is an attenuated mutant of Mycobacterium bovis that was isolated after serial subcultures, yet the functional basis for this attenuation has never been elucidated. A single region (RD1), which is absent in all BCG substrains, was deleted from virulent M. bovis and Mycobacterium tuberculosis strains, and the resulting DeltaRD1 mutants were significantly attenuated for virulence in both immunocompromised and immunocompetent mice. The M. tuberculosis DeltaRD1 mutants were also shown to protect mice against aerosol challenge, in a similar manner to BCG. Interestingly, the DeltaRD1 mutants failed to cause cytolysis of pneumocytes, a phenotype that had been previously used to distinguish virulent M. tuberculosis from BCG. A specific transposon mutation, which disrupts the Rv3874 Rv3875 (cfp-10 esat-6) operon of RD1, also caused loss of the cytolytic phenotype in both pneumocytes and macrophages. This mutation resulted in the attenuation of virulence in mice, as the result of reduced tissue invasiveness. Moreover, specific deletion of each transcriptional unit of RD1 revealed that three independent transcriptional units are required for virulence, two of which are involved in the secretion of ESAT-6 (6-kDa early secretory antigenic target). We conclude that the primary attenuating mechanism of bacillus Calmette-Guérin is the loss of cytolytic activity mediated by secreted ESAT-6, which results in reduced tissue invasiveness.
Figures
References
-
- Bloom, B. R. & Fine, P. (1994) in Tuberculosis Pathogenesis, Protection, and Control, ed. Bloom, B. R. (Am. Soc. Microbiol., Washington, DC).
-
- Calmette, A. & Guérin, C. (1909) C. R. Acad. Sci. 149, 716-718.
-
- Gheorghiu, M. (1996) in Vaccinia, Vaccination, and Vaccinology: Jenner, Pasteur and Their Successors, eds. Plotkin, S. A. & Fantini, B. (Elsevier, Paris), pp. 87-94.
-
- Calmette, A. & Guérin, C. (1905) Ann. Institut Pasteur 19, 601-618.
-
- Calmette, A. & Guérin, C. (1920) Ann. Inst. Pasteur 34, 553-561.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

