Genetically targeted adenovirus vector directed to CD40-expressing cells
- PMID: 14557622
- PMCID: PMC229360
- DOI: 10.1128/jvi.77.21.11367-11377.2003
Genetically targeted adenovirus vector directed to CD40-expressing cells
Abstract
The success of gene therapy depends on the specificity of transgene delivery by therapeutic vectors. The present study describes the use of an adenovirus (Ad) fiber replacement strategy for genetic targeting of the virus to human CD40, which is expressed by a variety of diseased tissues. The tropism of the virus was modified by the incorporation into its capsid of a protein chimera comprising structural domains of three different proteins: the Ad serotype 5 fiber, phage T4 fibritin, and the human CD40 ligand (CD40L). The tumor necrosis factor-like domain of CD40L retains its functional tertiary structure upon incorporation into this chimera and allows the virus to use CD40 as a surrogate receptor for cell entry. The ability of the modified Ad vector to infect CD40-positive dendritic cells and tumor cells with a high efficiency makes this virus a prototype of choice for the derivation of therapeutic vectors for the genetic immunization and targeted destruction of tumors.
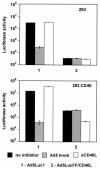
Figures








References
-
- Alemany, R., C. Balague, and D. T. Curiel. 2000. Replicative adenoviruses for cancer therapy. Nat. Biotechnol. 18:723-727. - PubMed
-
- Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. - PubMed
-
- Chroboczek, J., R. W. Ruigrok, and S. Cusack. 1995. Adenovirus fiber. Curr. Top. Microbiol. Immunol. 199:163-200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

