CpG DNA induces protective antiviral immune responses in Atlantic salmon (Salmo salar L.)
- PMID: 14557632
- PMCID: PMC229259
- DOI: 10.1128/jvi.77.21.11471-11479.2003
CpG DNA induces protective antiviral immune responses in Atlantic salmon (Salmo salar L.)
Abstract
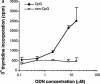
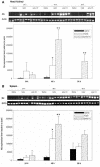
Oligodeoxynucleotides (ODN) containing unmethylated CpG dinucleotides within specific sequence contexts (CpG motifs) are detected, like bacterial or viral DNA, as a danger signal by the vertebrate immune system. CpG ODN show promise as vaccine adjuvants and immunoprotective agents in animal models. Here we report that pretreatment with CpG ODN in animals induces nonspecific protection against viral infection. A panel of different synthetic CpG ODN was tested for the in vitro effects in Atlantic salmon (Salmo salar L.) leukocytes. The ODN were tested for their capacity to stimulate proliferation of peripheral blood leukocytes and to induce production of interferon-like factors in head kidney leukocytes. These studies revealed that the sequence and number of the CpG motifs as well as the lengths of the ODN contribute to their stimulatory activity. ODN with the 6-mer CpG motif (5'-GTCGTT-3') showed the highest stimulatory activity and were shown to induce protection against infectious pancreatic necrosis virus when injected in Atlantic salmon. Expression of the Mx transcript, as an indicator of alpha/beta interferon induction, was induced in the CpG-injected fish. These results suggest that CpG DNA in fish induces early, nonspecific antiviral protection.
Figures



Similar articles
-
CpG oligodeoxynucleotides and plasmid DNA stimulate Atlantic salmon (Salmo salar L.) leucocytes to produce supernatants with antiviral activity.Dev Comp Immunol. 2001 May;25(4):313-21. doi: 10.1016/s0145-305x(00)00068-9. Dev Comp Immunol. 2001. PMID: 11246071
-
Stimulation of type I IFN activity in Atlantic salmon (Salmo salar L.) leukocytes: synergistic effects of cationic proteins and CpG ODN.Fish Shellfish Immunol. 2006 Apr;20(4):503-18. doi: 10.1016/j.fsi.2005.06.009. Epub 2005 Aug 22. Fish Shellfish Immunol. 2006. PMID: 16115781
-
Expression kinetics of interferon and interferon-induced genes in Atlantic salmon (Salmo salar) following infection with infectious pancreatic necrosis virus and infectious salmon anaemia virus.Fish Shellfish Immunol. 2007 Mar;22(3):230-41. doi: 10.1016/j.fsi.2006.05.004. Epub 2006 May 23. Fish Shellfish Immunol. 2007. PMID: 16806972
-
Strategies for enhancing the immunostimulatory effects of CpG oligodeoxynucleotides.J Control Release. 2004 May 31;97(1):1-17. doi: 10.1016/j.jconrel.2004.02.022. J Control Release. 2004. PMID: 15147800 Review.
-
A review of CpGs and their relevance to aquaculture.Vet Immunol Immunopathol. 2006 Aug 15;112(3-4):87-101. doi: 10.1016/j.vetimm.2006.03.015. Epub 2006 Jun 5. Vet Immunol Immunopathol. 2006. PMID: 16750571 Review.
Cited by
-
CpG-ODN induced antimicrobial immunity in neonatal chicks involves a substantial shift in serum metabolic profiles.Sci Rep. 2021 Apr 27;11(1):9028. doi: 10.1038/s41598-021-88386-2. Sci Rep. 2021. PMID: 33907214 Free PMC article.
-
Transcriptome profiles associated to VHSV infection or DNA vaccination in turbot (Scophthalmus maximus).PLoS One. 2014 Aug 6;9(8):e104509. doi: 10.1371/journal.pone.0104509. eCollection 2014. PLoS One. 2014. PMID: 25098168 Free PMC article.
-
Effectiveness of dietary heat-killed Bacillus subtilis harboring plasmid containing 60 copies of CpG-ODN 1668 against Vibrio harveyi in Penaeus vannamei.Vet Res Commun. 2024 Feb;48(1):85-101. doi: 10.1007/s11259-023-10182-2. Epub 2023 Aug 2. Vet Res Commun. 2024. PMID: 37530963
-
Innate immune defenses induced by CpG do not promote vaccine-induced protection against foot-and-mouth disease virus in pigs.Clin Vaccine Immunol. 2009 Aug;16(8):1151-7. doi: 10.1128/CVI.00018-09. Epub 2009 Jun 24. Clin Vaccine Immunol. 2009. PMID: 19553550 Free PMC article.
-
CpG-ODN Induces a Dose-Dependent Enrichment of Immunological Niches in the Spleen and Lungs of Neonatal Chicks That Correlates with the Protective Immunity against Escherichia coli.J Immunol Res. 2020 Jan 13;2020:2704728. doi: 10.1155/2020/2704728. eCollection 2020. J Immunol Res. 2020. PMID: 32411791 Free PMC article.
References
-
- Ballas, Z. K., W. L. Rasmussen, and A. M. Krieg. 1996. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J. Immunol. 157:1840-1845. - PubMed
-
- Biron, C. A. 1998. Role of early cytokines, including alpha and beta interferons (IFN-α/β), in innate and adaptive immune responses to viral infections. Semin. Immunol. 10:383-390. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

