Evidence against an essential role of COPII-mediated cargo transport to the endoplasmic reticulum-Golgi intermediate compartment in the formation of the primary membrane of vaccinia virus
- PMID: 14557660
- PMCID: PMC229368
- DOI: 10.1128/jvi.77.21.11754-11766.2003
Evidence against an essential role of COPII-mediated cargo transport to the endoplasmic reticulum-Golgi intermediate compartment in the formation of the primary membrane of vaccinia virus
Abstract
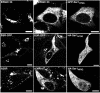
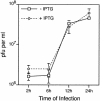
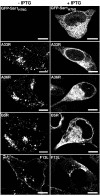
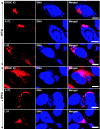
Vaccinia virus assembles two distinct lipoprotein membranes. The primary membrane contains nonglycosylated proteins, appears as crescents in the cytoplasm, and delimits immature and mature intracellular virions. The secondary or wrapping membrane contains glycoproteins, is derived from virus-modified trans-Golgi or endosomal cisternae, forms a loose coat around some intracellular mature virions, and becomes the envelope of extracellular virions. Although the mode of formation of the wrapping membrane is partially understood, we know less about the primary membrane. Recent reports posit that the primary membrane originates from the endoplasmic reticulum-Golgi intermediate compartment (ERGIC). According to this model, viral primary membrane proteins are cotranslationally inserted into the ER and accumulate in the ERGIC. To test the ERGIC model, we employed Sar1(H79G), a dominant negative form of the Sar1 protein, which is an essential component of coatomer protein II (COPII)-mediated cargo transport from the ER to the ERGIC and other post-ER compartments. Overexpression of Sar1(H79G) by transfection or by a novel recombinant vaccinia virus with an inducible Sar1(H79G) gene resulted in retention of ERGIC 53 in the ER but did not interfere with localization of viral primary membrane proteins in factory regions or with formation of viral crescent membranes and infectious intracellular mature virions. Wrapping of intracellular mature virions and formation of extracellular virions did not occur, however, because some proteins that are essential for the secondary membrane were retained in the ER as a consequence of Sar1(H79G) overexpression. Our data argue against an essential role of COPII-mediated cargo transport and the ERGIC in the formation of the viral primary membrane. Instead, viral membranes may be derived directly from the ER or by a novel mechanism.
Figures






Similar articles
-
COPII coat assembly and selective export from the endoplasmic reticulum.J Biochem. 2004 Dec;136(6):755-60. doi: 10.1093/jb/mvh184. J Biochem. 2004. PMID: 15671485 Review.
-
Maintenance of Golgi structure and function depends on the integrity of ER export.J Cell Biol. 2001 Nov 12;155(4):557-70. doi: 10.1083/jcb.200107045. Epub 2001 Nov 12. J Cell Biol. 2001. PMID: 11706049 Free PMC article.
-
Sequential coupling between COPII and COPI vesicle coats in endoplasmic reticulum to Golgi transport.J Cell Biol. 1995 Nov;131(4):875-93. doi: 10.1083/jcb.131.4.875. J Cell Biol. 1995. PMID: 7490291 Free PMC article.
-
TFG facilitates outer coat disassembly on COPII transport carriers to promote tethering and fusion with ER-Golgi intermediate compartments.Proc Natl Acad Sci U S A. 2017 Sep 12;114(37):E7707-E7716. doi: 10.1073/pnas.1709120114. Epub 2017 Aug 29. Proc Natl Acad Sci U S A. 2017. PMID: 28851831 Free PMC article.
-
COPII-mediated trafficking at the ER/ERGIC interface.Traffic. 2019 Jul;20(7):491-503. doi: 10.1111/tra.12654. Epub 2019 May 30. Traffic. 2019. PMID: 31059169 Free PMC article. Review.
Cited by
-
Association of the vaccinia virus A11 protein with the endoplasmic reticulum and crescent precursors of immature virions.J Virol. 2013 Sep;87(18):10195-206. doi: 10.1128/JVI.01601-13. Epub 2013 Jul 17. J Virol. 2013. PMID: 23864611 Free PMC article.
-
Deep-etch EM reveals that the early poxvirus envelope is a single membrane bilayer stabilized by a geodetic "honeycomb" surface coat.J Cell Biol. 2005 Apr 25;169(2):269-83. doi: 10.1083/jcb.200412169. J Cell Biol. 2005. PMID: 15851517 Free PMC article.
-
Vaccinia virus A6 is essential for virion membrane biogenesis and localization of virion membrane proteins to sites of virion assembly.J Virol. 2012 May;86(10):5603-13. doi: 10.1128/JVI.00330-12. Epub 2012 Mar 7. J Virol. 2012. PMID: 22398288 Free PMC article.
-
Lipid membranes in poxvirus replication.Viruses. 2010 Apr;2(4):972-986. doi: 10.3390/v2040972. Epub 2010 Apr 6. Viruses. 2010. PMID: 21994664 Free PMC article.
-
Poxvirus membrane biogenesis.Virology. 2015 May;479-480:619-26. doi: 10.1016/j.virol.2015.02.003. Epub 2015 Feb 26. Virology. 2015. PMID: 25728299 Free PMC article. Review.
References
-
- Barlowe, C., L. Orci, T. Yeung, M. Hosobuchi, S. Hamamoto, N. Salama, M. F. Rexach, M. Ravazzola, M. Amherdt, and R. Schekman. 1994. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 77:895-907. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

