Active borna disease virus polymerase complex requires a distinct nucleoprotein-to-phosphoprotein ratio but no viral X protein
- PMID: 14557662
- PMCID: PMC229352
- DOI: 10.1128/jvi.77.21.11781-11789.2003
Active borna disease virus polymerase complex requires a distinct nucleoprotein-to-phosphoprotein ratio but no viral X protein
Abstract
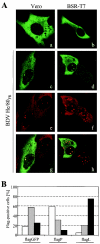
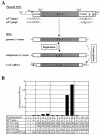
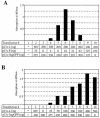
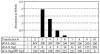
Analysis of the composition and regulation of the Borna disease virus (BDV) polymerase complex has so far been limited by the lack of a functional assay. To establish such an assay on the basis of an artificial minigenome, we constructed expression vectors encoding either nucleoprotein (N), phosphoprotein (P), X protein, or polymerase (L) of BDV under the control of the chicken beta-actin promoter. A Flag-tagged version of L colocalized with virus-encoded N and P in characteristic nuclear dots of BDV-infected cells and increased viral N-protein levels in persistently infected Vero cells. Vector-driven expression of L, N, and P in BSR-T7 cells together with a negative-sense BDV minigenome carrying a chloramphenicol acetyltransferase (CAT) reporter gene resulted in efficient synthesis of CAT protein. Induction of CAT protein synthesis strongly depended on a 10- to 30-fold molar excess of the N-encoding plasmid over the P-encoding plasmid. Cotransfection of even small amounts of plasmid encoding the viral X protein reduced CAT synthesis to background levels. Thus, the N-to-P stoichiometry seems to play a central role in the regulation of the BDV polymerase complex. Our data further suggest a negative regulatory function for the X protein of BDV.
Figures
References
-
- Billich, C., C. Sauder, R. Frank, S. Herzog, K. Bechter, K. Takahashi, H. Peters, P. Staeheli, and M. Schwemmle. 2002. High-avidity human serum antibodies recognizing linear epitopes of Borna disease virus proteins. Biol. Psychiatry 51:979-987. - PubMed
-
- Buchholz, U. J., S. Finke, and K. K. Conzelmann. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73:251-259. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

