DNA-mediated charge transport for DNA repair
- PMID: 14559969
- PMCID: PMC240652
- DOI: 10.1073/pnas.2035257100
DNA-mediated charge transport for DNA repair
Erratum in
- Proc Natl Acad Sci U S A. 2004 Mar 30;101(13):4718
Abstract
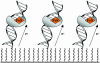
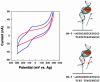
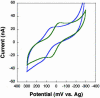
MutY, like many DNA base excision repair enzymes, contains a [4Fe4S]2+ cluster of undetermined function. Electrochemical studies of MutY bound to a DNA-modified gold electrode demonstrate that the [4Fe4S] cluster of MutY can be accessed in a DNA-mediated redox reaction. Although not detectable without DNA, the redox potential of DNA-bound MutY is approximately 275 mV versus NHE, which is characteristic of HiPiP iron proteins. Binding to DNA is thus associated with a change in [4Fe4S]3+/2+ potential, activating the cluster toward oxidation. Given that DNA charge transport chemistry is exquisitely sensitive to perturbations in base pair structure, such as mismatches, we propose that this redox process of MutY bound to DNA exploits DNA charge transport and provides a DNA signaling mechanism to scan for mismatches and lesions in vivo.
Figures


References
-
- Guan, Y., Manuel, R. C., Arvai, A. S., Parikh, S. S., Mol, C. D., Miller, J. H., Lloyd, S. & Tainer, J. A. (1998) Nat. Struct. Biol. 5 1058–1064. - PubMed
-
- Cunningham, R. P., Asahara, H., Bank, J. F., Scholes, C. P., Salerno, J. C., Surerus, K., Munck, E., McCracken, J., Peisach, J. & Emptage, M. H. (1989) Biochemistry 28 4450–4455. - PubMed
-
- Pomposiello, P. J. & Demple, B. (2001) Trends Biotechnol. 19 109–114. - PubMed
-
- Unden, G. & Bongaerts, J. (1997) Biochim. Biophys. Acta 1320 217–222. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

