Miz1 is required for early embryonic development during gastrulation
- PMID: 14560010
- PMCID: PMC207589
- DOI: 10.1128/MCB.23.21.7648-7657.2003
Miz1 is required for early embryonic development during gastrulation
Abstract
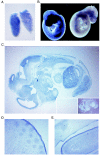
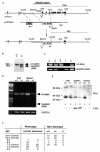
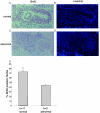
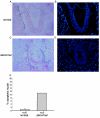
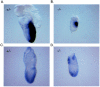
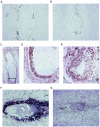
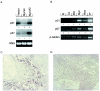
Miz1 is a member of the POZ domain/zinc finger transcription factor family. In vivo, Miz1 forms a complex with the Myc oncoprotein and recruits Myc to core promoter elements. Myc represses transcription through Miz1 binding sites. We now show that the Miz1 gene is ubiquitously expressed during mouse embryogenesis. In order to elucidate the physiological function of Miz1, we have deleted the mouse Miz1 gene by homologous recombination. Miz1(+/-) mice are indistinguishable from wild-type animals; in contrast, Miz1(-/-) embryos are not viable. They are severely retarded in early embryonic development and do not undergo normal gastrulation. Expression of Goosecoid and Brachyury is detectable in Miz1(-/-) embryos, suggesting that Miz1 is not required for signal transduction by Nodal. Expression of p21Cip1, a target gene of Miz1 is unaltered; in contrast, expression of p57Kip2, another target gene of Miz1 is absent in Miz1(-/-) embryos. Miz1(-/-) embryos succumb to massive apoptosis of ectodermal cells around day 7.5 of embryonic development. Our results show that Miz1 is required for early embryonic development during gastrulation.
Figures

References
-
- Amati, B., M. W. Brooks, N. Levy, T. D. Littlewood, G. I. Evan, and H. Land. 1993. Oncogenic activity of the c-Myc protein requires dimerization with Max. Cell 72:233-245. - PubMed
-
- Arnold, I., and F. M. Watt. 2001. c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and differentiation of their progeny. Curr. Biol. 11:558-568. - PubMed
-
- Bancroft, J. D., and A. Stevens. 1990. Theory and practice of histological techniques, 3rd ed. Churchill Livingstone, Edinburgh, United Kingdom.
-
- Bardwell, V. J., and R. Treisman. 1994. The POZ domain: a conserved protein-protein interaction motif. Genes Dev. 8:1664-1677. - PubMed
-
- Belo, J. A., T. Bouwmeester, L. Leyns, N. Kertesz, M. Gallo, M. Follettie, and E. M. De Robertis. 1997. Cerberus-like is a secreted factor with neutralizing activity expressed in the anterior primitive endoderm of the mouse gastrula. Mech. Dev. 68:45-57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
