Neurogenesis and brain injury: managing a renewable resource for repair
- PMID: 14561695
- PMCID: PMC213498
- DOI: 10.1172/JCI20098
Neurogenesis and brain injury: managing a renewable resource for repair
Abstract
The brain shows limited ability to repair itself, but neurogenesis in certain areas of the adult brain suggests that neural stem cells may be used for structural brain repair. It will be necessary to understand how neurogenesis in the adult brain is regulated to develop strategies that harness neural stem cells for therapeutic use.
Figures
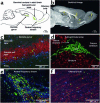
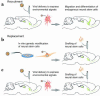

References
-
- Ramon y Cajal, S. 1928. Degeneration and regeneration of the nervous system. Volume 2. Haffner Publishing Co. New York, New York, USA. p. 750.
-
- Peterson DA. Stem cells in brain plasticity and repair. Curr. Opin. Pharmacol. 2002;2:34–42. - PubMed
-
- Kornack DR, Rakic P. Cell proliferation without neurogenesis in adult primate neocortex. Science. 2001;294:2127–2130. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

