Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors
- PMID: 14561699
- PMCID: PMC213487
- DOI: 10.1172/JCI18549
Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors
Abstract
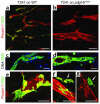
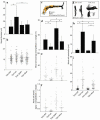
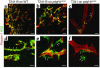
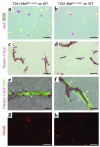
Tumor-infiltrating blood vessels deviate morphologically and biochemically from normal vessels, raising the prospect of selective pharmacological targeting. Current antiangiogenic approaches focus mainly on endothelial cells, but recent data imply that targeting pericytes may provide additional benefits. Further development of these concepts will require deeper insight into mechanisms of pericyte recruitment and function in tumors. Here, we applied genetic tools to decipher the function of PDGF-B and PDGF-Rbeta in pericyte recruitment in a mouse fibrosarcoma model. In tumors transplanted into PDGF-B retention motif-deficient (pdgf-b(ret/ret)) mice, pericytes were fewer and were partially detached from the vessel wall, coinciding with increased tumor vessel diameter and hemorrhaging. Transgenic PDGF-B expression in tumor cells was able to increase the pericyte density in both WT and pdgf-b(ret/ret) mice but failed to correct the pericyte detachment in pdgf-b(ret/ret) mice. Coinjection of exogenous pericytes and tumor cells showed that pericytes require PDGF-Rbeta for recruitment to tumor vessels, whereas endothelial PDGF-B retention is indispensable for proper integration of pericytes in the vessel wall. Our data support the notion that pericytes serve an important function in tumor vessels and highlight PDGF-B and PDGF-Rbeta as promising molecular targets for therapeutic intervention.
Figures



Comment in
-
What brings pericytes to tumor vessels?J Clin Invest. 2003 Oct;112(8):1134-6. doi: 10.1172/JCI20087. J Clin Invest. 2003. PMID: 14561696 Free PMC article.
References
-
- Folkman J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. - PubMed
-
- Yuan F, et al. Vascular permeability and microcirculation of gliomas and mammary carcinomas transplanted in rat and mouse cranial windows. Cancer Res. 1994;54:4564–4568. - PubMed
-
- Yuan F, et al. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 1995;55:3752–3756. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

