A homozygous mutation in HESX1 is associated with evolving hypopituitarism due to impaired repressor-corepressor interaction
- PMID: 14561704
- PMCID: PMC213489
- DOI: 10.1172/JCI18589
A homozygous mutation in HESX1 is associated with evolving hypopituitarism due to impaired repressor-corepressor interaction
Abstract
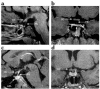
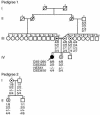
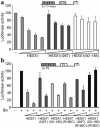
The paired-like homeobox gene expressed in embryonic stem cells Hesx1/HESX1 encodes a developmental repressor and is expressed in early development in a region fated to form the forebrain, with subsequent localization to Rathke's pouch, the primordium of the anterior pituitary gland. Mutations within the gene have been associated with septo-optic dysplasia, a constellation of phenotypes including eye, forebrain, and pituitary abnormalities, or milder degrees of hypopituitarism. We identified a novel homozygous nonconservative missense mutation (I26T) in the critical Engrailed homology repressor domain (eh1) of HESX1, the first, to our knowledge, to be described in humans, in a girl with evolving combined pituitary hormone deficiency born to consanguineous parents. Neuroimaging revealed a thin pituitary stalk with anterior pituitary hypoplasia and an ectopic posterior pituitary, but no midline or optic nerve abnormalities. This I26T mutation did not affect the DNA-binding ability of HESX1 but led to an impaired ability to recruit the mammalian Groucho homolog/Transducin-like enhancer of split-1 (Gro/TLE1), a crucial corepressor for HESX1, thereby leading to partial loss of repression. Thus, the novel pituitary phenotype highlighted here appears to be a specific consequence of the inability of HESX1 to recruit Groucho-related corepressors, suggesting that other molecular mechanisms govern HESX1 function in the forebrain.
Figures




References
-
- Schwind JL. The development of the hypophysis cerebri of the albino rat. Am. J. Anat. 1928;41:295–315.
-
- Kaufman, M.H. 1992. An atlas of mouse development. Academic Press, Elsevier Science. Oxford, United Kingdom. 39–210.
-
- Dattani MT, et al. Mutations in the homeobox gene HESX1/Hesx1 associated with septo-optic dysplasia in human and mouse. Nat. Genet. 1998;19:125–133. - PubMed
-
- Martinez-Barbera JP, Rodriguez TA, Beddington RS. The homeobox gene Hesx1 is required in the anterior neural ectoderm for normal forebrain formation. Dev. Biol. 2000;223:422–430. - PubMed
-
- Thomas PQ, et al. Heterozygous HESX1 mutations associated with isolated congenital pituitary hypoplasia and septo-optic dysplasia. Hum. Mol. Genet. 2001;10:39–45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

