Membrane properties of type II spiral ganglion neurones identified in a neonatal rat cochlear slice
- PMID: 14561834
- PMCID: PMC2343372
- DOI: 10.1111/j.1469-7793.2003.00525.x
Membrane properties of type II spiral ganglion neurones identified in a neonatal rat cochlear slice
Abstract
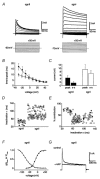
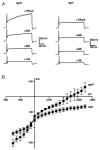
Neuro-anatomical studies in the mammalian cochlea have previously identified a subpopulation of approximately 5 % of primary auditory neurones, designated type II spiral ganglion neurones (sgnII). These neurones project to outer hair cells and their supporting cells, within the 'cochlear amplifier' region. Physiological characterization of sgnII has proven elusive. Whole-cell patch clamp of spiral ganglion neurones in P7-P10 rat cochlear slices provided functional characterization of sgnII, identified by biocytin or Lucifer yellow labelling of their peripheral neurite projections (outer spiral fibres) subsequent to electrophysiological characterisation. SgnII terminal fields comprised multiple outer hair cells and supporting cells, located up to 370 mum basal to their soma. SgnII firing properties were defined by rapidly inactivating A-type-like potassium currents that suppress burst firing of action potentials. Type I spiral ganglion neurones (sgnI), had shorter radial projections to single inner hair cells and exhibited larger potassium currents with faster activation and slower inactivation kinetics, compatible with the high temporal firing fidelity seen in auditory nerve coding. Based on these findings, sgnII may be identified in future by the A-type current. Glutamate-gated somatic currents in sgnII were more potentiated by cyclothiazide than those in sgnI, suggesting differential AMPA receptor expression. ATP-activated desensitising inward currents were comparable in sgn II and sgnI. These data support a role for sgnII in providing integrated afferent feedback from the cochlear amplifier.
Figures


References
-
- Adamson CL, Reid MA, Mo ZL, Bowne-English, Davis RL. Firing features and potassium channel content of murine spiral ganglion neurons vary with cochlear location. J Comp Neurol. 2002;447:331–350. - PubMed
-
- Ashmore JF, Kolston PJ. Hair cell based amplification in the cochlea. Curr Op Neurobiol. 1994;4:503–508. - PubMed
-
- Berglund AM, Brown MC. Central trajectories of type II spiral ganglion cells from various cochlear regions in mice. Hear Res. 1994;75:121–130. - PubMed
-
- Berglund AM, Ryugo DK. Hair cell innervation by spiral ganglion neurons in the mouse. J Comp Neurol. 1987;255:560–570. - PubMed
-
- Brown MC. Morphology of labeled afferent fibers in the guinea pig cochlea. J Comp Neurol. 1987;260:591–604. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

