Histone deacetylase inhibition by sodium butyrate chemotherapy ameliorates the neurodegenerative phenotype in Huntington's disease mice
- PMID: 14561870
- PMCID: PMC6740577
- DOI: 10.1523/JNEUROSCI.23-28-09418.2003
Histone deacetylase inhibition by sodium butyrate chemotherapy ameliorates the neurodegenerative phenotype in Huntington's disease mice
Abstract
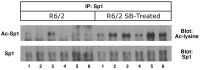
The precise cause of neuronal death in Huntington's disease (HD) is unknown. Although no single specific protein-protein interaction of mutant huntingtin has emerged as the pathologic trigger, transcriptional dysfunction may contribute to the neurodegeneration observed in HD. Pharmacological treatment using the histone deacetylase inhibitor sodium butyrate to modulate transcription significantly extended survival in a dose-dependent manner, improved body weight and motor performance, and delayed the neuropathological sequelae in the R6/2 transgenic mouse model of HD. Sodium butyrate also increased histone and Specificity protein-1 acetylation and protected against 3-nitropropionic acid neurotoxicity. Microarray analysis showed increased expression of alpha- and beta-globins and MAP kinase phosphatase-1 in sodium butyrate-treated R6/2 mice, indicative of improved oxidative phosphorylation and transcriptional regulation. These findings strengthen the hypothesis that transcriptional dysfunction plays a role in the pathogenesis of HD and suggest that therapies aimed at modulating transcription may target early pathological events and provide clinical benefits to HD patients.
Figures








References
-
- Andreassen OA, Ferrante RJ, Huang HM, Dedeoglu A, Park L, Ferrante KL, Kwon J, Borchelt DR, Ross CA, Gibson GE, Beal MF ( 2001a) Dichloroacetate exerts therapeutic effects in transgenic mouse models of Huntington's disease. Ann Neurol 50: 112-117. - PubMed
-
- Andreassen OA, Ferrante RJ, Dedeoglu A, Beal MF ( 2001b) Lipoic acid improves survival in transgenic mouse models of Huntington's disease. NeuroReport 12: 3371-3374. - PubMed
-
- Beal MF ( 2000) Energetics in the pathogenesis of neurodegenerative diseases. Trends Neurosci 7: 298-304. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
