Early phagosomes in dendritic cells form a cellular compartment sufficient for cross presentation of exogenous antigens
- PMID: 14561893
- PMCID: PMC240714
- DOI: 10.1073/pnas.1735556100
Early phagosomes in dendritic cells form a cellular compartment sufficient for cross presentation of exogenous antigens
Abstract
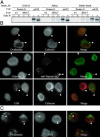
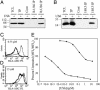
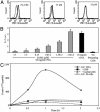
Conventionally, MHC class I-restricted antigen (Ag) processing requires the action of the multimolecular peptide-loading complex within the endoplasmic reticulum (ER). Here we show that early phagosomes from human dendritic cells (DCs) contain the peptide-loading complex, incorporating MHC class I, beta2 microglobulin, transporter associated with Ag processing (TAP), calreticulin, tapasin, and ERp57. Antigenic peptides could be translocated into purified phagosomes by TAP and loaded onto cognate class I molecules, inducing their specific dissociation from the loading complex. Endoglycosidase H-sensitive class I molecules were detected at the DC cell surface, suggesting that these molecules traffic there directly from phagosomes. Macropinocytosis also allowed internalized soluble Ags access to an ER-like compartment containing the class I loading complex. Blockade of TAP by endocytosis of a soluble derivative of human cytomegalovirus protein US6 confirmed that, although retrotranslocation into the cytosol is critical for processing, efficient association of class I molecules with peptides derived from exogenous Ags occurs within a compartment directly accessible to internalized proteins. Together, this evidence suggests that early phagosomes and pinosomes facilitate cross presentation of exogenous Ags by DCs.
Figures



References
-
- Lankat-Buttgereit, B. & Tampe, R. (2002) Physiol. Rev. 82 187–204. - PubMed
-
- Radcliffe, C. M., Diedrich, G., Harvey, D. J., Dwek, R. A., Cresswell, P. & Rudd, P. M. (2002) J. Biol. Chem. 277 46415–46423. - PubMed
-
- Dick, T. P., Bangia, N., Peaper, D. R. & Cresswell, P. (2002) Immunity 16 87–98. - PubMed
-
- Serwold, T., Gonzalez, F., Kim, J., Jacob, R. & Shastri, N. (2002) Nature 419 480–483. - PubMed
-
- Saric, T., Chang, S. C., Hattori, A., York, I. A., Markant, S., Rock, K. L., Tsujimoto, M. & Goldberg, A. L. (2002) Nat. Immunol. 3 1169–1176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

