Interaction between the Alzheimer's survival peptide humanin and insulin-like growth factor-binding protein 3 regulates cell survival and apoptosis
- PMID: 14561895
- PMCID: PMC240741
- DOI: 10.1073/pnas.2135111100
Interaction between the Alzheimer's survival peptide humanin and insulin-like growth factor-binding protein 3 regulates cell survival and apoptosis
Abstract
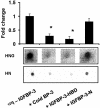
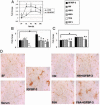
Insulin-like growth factor-binding protein-3 (IGFBP-3) regulates IGF bioactivity and also independently modulates cell growth and survival. By using a yeast two-hybrid screen to identify IGFBP-3-interacting proteins, we cloned humanin (HN) as an IGFBP-3-binding partner. HN is a 24-aa peptide that has been shown to specifically inhibit neuronal cell death induced by familial Alzheimer's disease mutant genes and amyloid-beta (Abeta). The physical interaction of HN with IGFBP-3 was determined to be of high affinity and specificity and was confirmed by yeast mating, displaceable pull-down experiments with (His)-6-tagged HN, and ligand blot experiments. Co-immunoprecipitation of IGFBP-3 and HN from mouse testes confirmed the interaction in vivo. In cross-linking experiments, HN bound IGFBP-3 but did not compete with IGF-I-IGFBP-3 binding; competitive ligand dot blot experiments revealed the 18-aa heparin-binding domain of IGFBP-3 as the binding site for HN. Alanine scanning determined that F6A-HN mutant does not bind IGFBP-3. HN but not F6A-HN inhibited IGFBP-3-induced apoptosis in human glioblastoma-A172. In contrast, HN did not suppress IGFBP-3 response in SH-SY5Y neuroblastoma and mouse cortical primary neurons. In primary neurons, IGFBP-3 markedly potentiated HN rescue ability from Abeta1-43 toxicity. In summary, we have identified an interaction between the survival peptide HN and IGFBP-3 that is pleiotrophic in nature and is capable of both synergistic and antagonistic interaction. This interaction may prove to be important in neurological disease processes and could provide important targets for drug development.
Figures

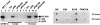

References
-
- Bredesen, D. E. (1995) Ann. Neurol. 38 839-851. - PubMed
-
- Conway, K. A., Baxter, E. W., Felsenstein, K. M. & Reitz, A. B. (2003) Curr. Pharm. Des. 9 427-447. - PubMed
-
- Vincent, A. M. & Feldman, E. L. (2002) Growth Horm. IGF Res. 12 193-197. - PubMed
-
- Carro, E., Trejo, J. L., Gomez-Isla, T., LeRoith, D. & Torres-Aleman, I. (2002) Nat. Med. 8 1390-1397. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

