Thrombospondin 1, a mediator of the antiangiogenic effects of low-dose metronomic chemotherapy
- PMID: 14561896
- PMCID: PMC240719
- DOI: 10.1073/pnas.2135406100
Thrombospondin 1, a mediator of the antiangiogenic effects of low-dose metronomic chemotherapy
Abstract
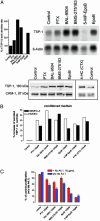
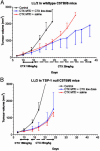
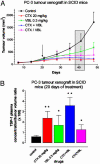
Chemotherapeutic drugs chronically administered to tumor-bearing mice, using a frequent schedule at doses substantially lower than the maximum tolerated dose (MTD) (i.e., metronomic dosing), can cause sustained and potent antiangiogenic effects by targeting the endothelial cells of newly growing tumor blood vessels. These effects appear to occur in the absence of an increase in the severity of side effects caused by destruction of other cell types normally sensitive to MTD chemotherapy, suggesting a marked and selective sensitivity of activated endothelial cells, the basis of which is unknown. Here we report that protracted exposure of endothelial cells in vitro to low concentrations of several different anticancer agents, including microtubule inhibitors and an alkylating agent, caused marked induction of gene and protein expression of TSP-1, a potent and endothelial-specific inhibitor of angiogenesis. Increases in circulating TSP-1 were also detected in the plasma of human tumor-bearing severe combined immunodeficient mice treated with metronomic low-dose cyclophosphamide. Most importantly, the antiangiogenic and antitumor effects of low-dose continuous cyclophosphamide were lost in TSP-1-null C57BL/6 mice, whereas, in contrast, these effects were retained by using a MTD schedule of the same drug. Taken together, the results implicate TSP-1 as a secondary mediator of the antiangiogenic effects of at least some low-dose metronomic chemotherapy regimens.
Figures

References
-
- Miller, K. D., Sweeney, C. J. & Sledge, G. W., Jr. (2001) J. Clin. Oncol. 19 1195–1206. - PubMed
-
- Eberhard, A., Kahlert, S., Goede, V., Hemmerlein, B., Plate, K. H. & Augustin, H. G. (2000) Cancer Res. 60 1388–1393. - PubMed
-
- Browder, T., Butterfield, C. E., Kraling, B. M., Marshall, B., O'Reilly, M. S. & Folkman, J. (2000) Cancer Res. 60 1878–1886. - PubMed
-
- Kerbel, R. S. (1991) BioEssays 13 31–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

