Influenza virus hemagglutinin concentrates in lipid raft microdomains for efficient viral fusion
- PMID: 14561897
- PMCID: PMC299746
- DOI: 10.1073/pnas.2235620100
Influenza virus hemagglutinin concentrates in lipid raft microdomains for efficient viral fusion
Abstract
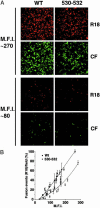
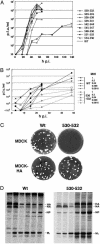
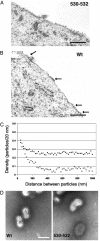
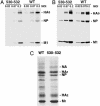
Lipid raft microdomains are enriched in sphingomyelin and cholesterol and function as platforms for signal transduction and as the site of budding of several enveloped viruses, including influenza virus. The influenza virus hemagglutinin (HA) glycoprotein, which mediates both viral-cell attachment and membrane fusion, associates intrinsically with lipid rafts. Residues in the HA transmembrane (TM) domain are important for raft association as sequence substitutions in the HA TM domain ablate HA association with rafts (nonraft HA). Cells expressing either WT or nonraft HA cause complete fusion (lipid mixing and content mixing) over widely varying HA expression levels. However, the number of fusion events measured for nonraft HA mutant protein at all HA surface densities was reduced to approximately 55% of the events for WT HA protein. Mutant influenza viruses were generated that contain the nonraft HA TM domain alterations. Electron microscopy experiments showed that WT HA was distributed at the cell surface in clusters of 200-280 nm in diameter, whereas nonraft HA was distributed mostly randomly at the plasma membrane. Nonraft HA virus showed reduced budding, contained reduced amounts of HA protein, was greatly reduced in infectivity, and exhibited decreased virus-membrane fusion activity. Cholesterol depletion of virus did not affect the ability of virions to cause either virus-cell lipid mixing or virus-mediated hemolysis, a surrogate for content mixing. Taken together, the data suggest that HA clusters in rafts to provide a sufficient concentration of HA in budding virus to mediate efficient virus-cell fusion.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

