Prognostic value of CA 19-9 levels in patients with inoperable adenocarcinoma of the pancreas treated with gemcitabine
- PMID: 14562009
- PMCID: PMC2394360
- DOI: 10.1038/sj.bjc.6601263
Prognostic value of CA 19-9 levels in patients with inoperable adenocarcinoma of the pancreas treated with gemcitabine
Abstract
Serum carbohydrate antigen 19-9 (CA 19-9) has been identified as a useful tumour marker for diagnosis of exocrine pancreatic carcinoma, but its value for evaluating the response to chemotherapy with gemcitabine is not clear. Tumour regression in pancreatic carcinoma is hard to determine due to massive desmoplastic tissue. Furthermore, objective tumour response does not automatically transcribe into better survival. Therefore, clinical benefit response, a composed parameter consisting of factors like performance status, pain, and body weight was integrated in evaluating tumour response. The aim of this prospective study was to evaluate the usefulness of serial CA 19-9 measurements as a biochemical response marker and an outcome prognostic parameter in patients with advanced pancreatic cancer receiving gemcitabine treatment. A total of 46 consecutive patients (median age 66 years) suffering from histologically proven locally advanced or metastatic adenocarcinoma of the exocrine pancreas were analysed. Gemcitabine was applied for a median of 23 courses (range 6-76). Two patients achieved an objective complete remission, five an objective partial remission (overall response, OR=15.2%), while objective stable disease was documented in 19 and objective progressive disease in 20 patients. Patients with a decrease of >20% of the baseline CA 19-9 level after 8 weeks of chemotherapy had a significantly better median survival than patients with a rise or a decline <20%. The response of CA 19-9 >20% during chemotherapy was the only independent predictor of survival in a multivariate analyses. In contrast, neither objective tumour response nor clinical benefit response showed this level of significance. In conclusion, kinetics of CA19-9 serum concentration serves as an early indicator of response to gemcitabine chemotherapy in advanced pancreatic cancer.
Figures
Similar articles
-
Prognostic value of serum CA19-9 in patients with advanced pancreatic cancer receiving gemcitabine based chemotherapy.Ai Zheng. 2009 Mar;28(3):286-91. Ai Zheng. 2009. PMID: 19619444
-
CA19-9: a pedictor of response in pancreatic cancer treated with gemcitabine and cisplatin.Anticancer Res. 1999 Jul-Aug;19(4A):2433-5. Anticancer Res. 1999. PMID: 10470171 Clinical Trial.
-
Decrease of CA 19-9 during chemotherapy with gemcitabine predicts survival time in patients with advanced pancreatic cancer.Br J Cancer. 2000 Mar;82(5):1013-6. doi: 10.1054/bjoc.1999.1035. Br J Cancer. 2000. PMID: 10737382 Free PMC article.
-
The role of second-line chemotherapy after gemcitabine failure in patients with advanced pancreatic cancer.Future Oncol. 2008 Feb;4(1):41-50. doi: 10.2217/14796694.4.1.41. Future Oncol. 2008. PMID: 18240999 Review.
-
Gemcitabine chemoresistance in pancreatic cancer: molecular mechanisms and potential solutions.Scand J Gastroenterol. 2009;44(7):782-6. doi: 10.1080/00365520902745039. Scand J Gastroenterol. 2009. PMID: 19214867 Review.
Cited by
-
First-line erlotinib and fixed dose-rate gemcitabine for advanced pancreatic cancer.World J Gastroenterol. 2013 Jul 28;19(28):4511-9. doi: 10.3748/wjg.v19.i28.4511. World J Gastroenterol. 2013. PMID: 23901226 Free PMC article. Clinical Trial.
-
Serum CA 19-9 as a Biomarker for Pancreatic Cancer-A Comprehensive Review.Indian J Surg Oncol. 2011 Jun;2(2):88-100. doi: 10.1007/s13193-011-0042-1. Epub 2011 Feb 17. Indian J Surg Oncol. 2011. PMID: 22693400 Free PMC article.
-
Second-line therapy for gemcitabine-pretreated advanced or metastatic pancreatic cancer.World J Gastroenterol. 2012 Mar 28;18(12):1357-64. doi: 10.3748/wjg.v18.i12.1357. World J Gastroenterol. 2012. PMID: 22493549 Free PMC article.
-
VEGF remains an interesting target in advanced pancreas cancer (APCA): results of a multi-institutional phase II study of bevacizumab, gemcitabine, and infusional 5-fluorouracil in patients with APCA.Ann Oncol. 2012 Nov;23(11):2812-2820. doi: 10.1093/annonc/mds134. Epub 2012 Jul 5. Ann Oncol. 2012. PMID: 22767582 Free PMC article. Clinical Trial.
-
C-reactive protein is a prognostic indicator in patients with perihilar cholangiocarcinoma.World J Gastroenterol. 2006 Sep 14;12(34):5495-500. doi: 10.3748/wjg.v12.i34.5495. World J Gastroenterol. 2006. PMID: 17006987 Free PMC article.
References
-
- Aapro MS, Martin C, Hatty S (1998) Gemcitabine–a safety review. Anticancer Drugs 9: 191–201 - PubMed
-
- Ahlgren JD (1996) Chemotherapy for pancreatic carcinoma. Cancer 78: 654–663 - PubMed
-
- Burris III HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD, Andersen JS, Tarassoff PG, Brown TD, Casper ES (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial A phase II trial of gemcitabine in patients with 5-FU-refractory pancreas cancer. J Clin Oncol 15: 2403–2413 - PubMed
-
- Cox D (1972) Regression models and life tables. J R Stat Soc [B] 34: 187–202
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

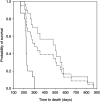
 ) during 8 weeks of chemotherapy with gemcitabine. Line
) during 8 weeks of chemotherapy with gemcitabine. Line  represents all patients.
represents all patients.