Adjuvant and neoadjuvant therapy for gastric cancer using epirubicin/cisplatin/5-fluorouracil (ECF) and alternative regimens before and after chemoradiation
- PMID: 14562013
- PMCID: PMC2394354
- DOI: 10.1038/sj.bjc.6601311
Adjuvant and neoadjuvant therapy for gastric cancer using epirubicin/cisplatin/5-fluorouracil (ECF) and alternative regimens before and after chemoradiation
Abstract
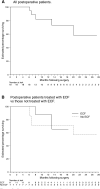
Chemoradiation is now used more commonly for gastric cancer following publication of the US Intergroup trial results that demonstrate an advantage to adjuvant postoperative chemoradiotherapy. However, there remain concerns regarding the toxicity of this treatment, the optimal chemotherapy regimen and the optimal method of radiotherapy delivery. In this prospective study, we evaluated the toxicity and feasibility of an alternative chemoradiation regimen to that used in the Intergroup trial. A total of 26 patients with adenocarcinoma of the stomach were treated with 3D-conformal radiation therapy to a dose of 45 Gy in 25 fractions with concurrent continuous infusional 5-fluorouracil (5-FU). The majority of patients received epirubicin, cisplatin and 5-FU (ECF) as the systemic component given before and after concurrent chemoradiation. The overall rates of observed grade 3 and 4 toxicities were 38 and 15%, respectively. GIT grade 3 toxicity was observed in 19% of patients, while haematologic grade 3 and 4 toxicities were observed in 23%. Our results suggest that this adjuvant regimen can be delivered safely and with acceptable toxicity. This regimen forms the basis of several new studies being developed for postoperative adjuvant therapy of gastric cancer.
Figures
Similar articles
-
Adjuvant chemoradiation for gastric cancer using epirubicin, cisplatin, and 5-fluorouracil before and after three-dimensional conformal radiotherapy with concurrent infusional 5-fluorouracil: a multicenter study of the Trans-Tasman Radiation Oncology Group.Int J Radiat Oncol Biol Phys. 2011 Mar 1;79(3):690-5. doi: 10.1016/j.ijrobp.2009.11.042. Epub 2010 May 14. Int J Radiat Oncol Biol Phys. 2011. PMID: 20472363 Clinical Trial.
-
Adjuvant Chemoradiotherapy With Epirubicin, Cisplatin, and Fluorouracil Compared With Adjuvant Chemoradiotherapy With Fluorouracil and Leucovorin After Curative Resection of Gastric Cancer: Results From CALGB 80101 (Alliance).J Clin Oncol. 2017 Nov 10;35(32):3671-3677. doi: 10.1200/JCO.2017.74.2130. Epub 2017 Oct 4. J Clin Oncol. 2017. PMID: 28976791 Free PMC article. Clinical Trial.
-
TOPGEAR: A Randomized, Phase III Trial of Perioperative ECF Chemotherapy with or Without Preoperative Chemoradiation for Resectable Gastric Cancer: Interim Results from an International, Intergroup Trial of the AGITG, TROG, EORTC and CCTG.Ann Surg Oncol. 2017 Aug;24(8):2252-2258. doi: 10.1245/s10434-017-5830-6. Epub 2017 Mar 23. Ann Surg Oncol. 2017. PMID: 28337660 Clinical Trial.
-
Evolving role of chemoradiation in the adjuvant treatment of gastric cancer.Expert Rev Anticancer Ther. 2004 Aug;4(4):585-94. doi: 10.1586/14737140.4.4.585. Expert Rev Anticancer Ther. 2004. PMID: 15270662 Review.
-
[Efficacy of current adjuvant and neoadjuvant therapeutic concepts in gastric cancer?].Zentralbl Chir. 2006 Apr;131(2):121-5. doi: 10.1055/s-2006-921537. Zentralbl Chir. 2006. PMID: 16612778 Review. German.
Cited by
-
Tumor blood supply may predict neoadjuvant chemotherapy response and survival in patients with gastric cancer.J Int Med Res. 2019 Jun;47(6):2524-2532. doi: 10.1177/0300060519845491. Epub 2019 May 1. J Int Med Res. 2019. PMID: 31039658 Free PMC article.
-
Survival after gastric adenocarcinoma resection: eighteen-year experience at a single institution.J Gastrointest Surg. 2005 May-Jun;9(5):718-25. doi: 10.1016/j.gassur.2004.12.002. J Gastrointest Surg. 2005. PMID: 15862270
-
Four consecutive multicenter phase II trials of adjuvant chemoradiation in patients with completely resected high-risk gastric cancer: the experience of the German AIO/ARO/CAO group.J Cancer Res Clin Oncol. 2009 Feb;135(2):163-72. doi: 10.1007/s00432-008-0463-6. Epub 2008 Sep 30. J Cancer Res Clin Oncol. 2009. PMID: 18825411 Free PMC article.
-
Safety and Feasibility of Carboplatin and Paclitaxel followed by Fluoropyrimidine Analogs and Radiation as Adjuvant Therapy for Gastric Cancer.Case Rep Oncol. 2009 Nov 21;2(3):220-228. doi: 10.1159/000250082. Case Rep Oncol. 2009. PMID: 20737041 Free PMC article.
-
Adjuvant chemoradiation for gastric cancer with infusional 5-fluorouracil and cisplatin: a phase I study.Curr Oncol. 2010 Aug;17(4):34-41. doi: 10.3747/co.v17i4.521. Curr Oncol. 2010. PMID: 20697512 Free PMC article.
References
-
- Beer M, Cocconi G, Ceci G, Varini M, Cavalli F (1983) A phase II study of cisplatin in advanced gastric cancer. Eur J Cancer Clin Oncol 19: 717–720 - PubMed
-
- Bonenkamp JJ, Hermans J, Sasako M, van_de_Velde CJ (1999) Extended lymph-node dissection for gastric cancer. Dutch Gastric Cancer Group. N Engl J Med 340: 908–914 - PubMed
-
- Cersosimo RJ, Hong WK (1986) Epirubicin: a review of the pharmacology, clinical activity, and adverse effects of an adriamycin analogue. J Clin Oncol 4: 425–439 - PubMed
-
- Chu JC, Solin LJ, Hwang CC, Kessler H, Hanks GE (1992) Three-dimensional dosimetric comparison of radiation therapy treatment planning of the pancreas. Med Dosim 17: 199–203 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

