Oxaliplatin- or irinotecan-based chemotherapy for metastatic colorectal cancer in the elderly
- PMID: 14562014
- PMCID: PMC2394343
- DOI: 10.1038/sj.bjc.6601310
Oxaliplatin- or irinotecan-based chemotherapy for metastatic colorectal cancer in the elderly
Abstract
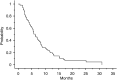
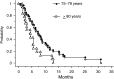
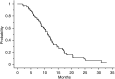
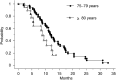
The tolerance and efficacy of oxaliplatin and irinotecan for metastatic colorectal cancer are unknown in elderly patients. Methods. All consecutive patients over 74 years treated with oxaliplatin or irinotecan for metastatic colorectal cancer were enrolled. The tumour response was assessed every 2-3 months and toxicity was collected at each cycle according to World Health Organisation criteria. A total of 66 patients were enrolled from 12 centres. The median age was 78 years (range, 75-88 years); 39 patients had no severe comorbidity according to the Charlson score. In total, 44 and 22 patients received oxaliplatin or irinotecan, respectively, in combination with 5-fluororuracil+/-folinic acid or raltitrexed in 64 patients. A total of 545 chemotherapy cycles were administered in first (41%), second (51%) or third line (8%). A dose reduction occurred in 190 cycles (35%). Complete response, partial response and stabilisation occurred in 1.5, 20 and 47% of patients, respectively. The median time to progression and overall survival were 6.8 and 11.2 months in first line and 6.3 and 11.6 months in second line, respectively. Grade 3 and 4 toxicity occurred in 42% of patients: neutropenia 17%, diarrhoea 15%, neuropathy 11%, nausea and vomiting 8% and thrombopenia 6%. There was no treatment-related death. In selected elderly patients, chemotherapy with oxaliplatin or irinotecan is feasible with manageable toxicity.
Figures
Comment in
-
Oxaliplatin- or irinotecan-based chemotherapy for metastatic colorectal cancer in the elderly.Br J Cancer. 2004 May 17;90(10):2050-1; author reply 2051-2. doi: 10.1038/sj.bjc.6601805. Br J Cancer. 2004. PMID: 15138493 Free PMC article. No abstract available.
Similar articles
-
Oxaliplatin plus raltitrexed and leucovorin-modulated 5-fluorouracil i.v. bolus: a salvage regimen for colorectal cancer patients.Br J Cancer. 2002 Jun 17;86(12):1871-5. doi: 10.1038/sj.bjc.6600414. Br J Cancer. 2002. PMID: 12085178 Free PMC article. Clinical Trial.
-
Multicenter phase II trial of dose-fractionated irinotecan in patients with advanced colorectal cancer failing oxaliplatin-based first-line combination chemotherapy.Ann Oncol. 2001 Sep;12(9):1269-72. doi: 10.1023/a:1012240201462. Ann Oncol. 2001. PMID: 11697839 Clinical Trial.
-
Chemotherapy of metastatic colorectal cancer: fluorouracil plus folinic acid and irinotecan or oxaliplatin.Prescrire Int. 2005 Dec;14(80):230-3. Prescrire Int. 2005. PMID: 16400749
-
Irinotecan in metastatic colorectal cancer: dose intensification and combination with new agents, including biological response modifiers.Ann Oncol. 2003;14 Suppl 2:ii17-23. doi: 10.1093/annonc/mdg724. Ann Oncol. 2003. PMID: 12810453 Review.
-
Recent experience with oxaliplatin or irinotecan combined with 5-fluorouracil and leucovorin in the treatment of colorectal cancer.Semin Oncol. 2003 Aug;30(4 Suppl 15):40-6. doi: 10.1016/s0093-7754(03)00404-4. Semin Oncol. 2003. PMID: 14523794 Review.
Cited by
-
Phase II study of capecitabine and mitomycin C as first-line treatment in patients with advanced colorectal cancer.Br J Cancer. 2004 Aug 31;91(5):839-43. doi: 10.1038/sj.bjc.6602039. Br J Cancer. 2004. PMID: 15266319 Free PMC article. Clinical Trial.
-
Targeted agents: review of toxicity in the elderly metastatic colorectal cancer patients.Target Oncol. 2011 Dec;6(4):245-51. doi: 10.1007/s11523-011-0198-1. Epub 2011 Nov 9. Target Oncol. 2011. PMID: 22068891 Review.
-
XELOX (capecitabine plus oxaliplatin) as first-line treatment for elderly patients over 70 years of age with advanced colorectal cancer.Br J Cancer. 2006 Apr 10;94(7):969-75. doi: 10.1038/sj.bjc.6603047. Br J Cancer. 2006. PMID: 16552438 Free PMC article. Clinical Trial.
-
Oxaliplatin- or irinotecan-based chemotherapy for metastatic colorectal cancer in the elderly.Br J Cancer. 2004 May 17;90(10):2050-1; author reply 2051-2. doi: 10.1038/sj.bjc.6601805. Br J Cancer. 2004. PMID: 15138493 Free PMC article. No abstract available.
-
Folfirinox in elderly patients with pancreatic or colorectal cancer-tolerance and efficacy.World J Gastroenterol. 2016 Nov 14;22(42):9378-9386. doi: 10.3748/wjg.v22.i42.9378. World J Gastroenterol. 2016. PMID: 27895425 Free PMC article.
References
-
- Andre T, Bensmaine MA, Louvet C, Francois E, Lucas V, Desseigne F, Beerblock K, Bouche O, Carola E, Merrouche Y, Morvan F, Dupont-Andre G, de Gramont A (1999) Multicenter phase II study of bimonthly high-dose leucovorin, fluorouracil infusion, and oxaliplatin for metastatic colorectal cancer resistant to the same leucovorin and fluorouracil regimen. J Clin Oncol 17: 3560–3568 - PubMed
-
- Beretta G, Bolina R, Cozzi C (1997) Should we consider the weekly (w) chemotherapy (CT) with fluorouracil (F)+racemic folinic acid (f) a standard treatment for advanced/metastatic carcinoma of digestive tract (DTC) in elderly patients (pts)? Proc Am Soc Clin Oncol 16: 259a
-
- Bray F, Sankila R, Ferlay J, Parkin DM (2002) Estimates of cancer incidence and mortality in Europe in 1995. Eur J Cancer 38: 99–166 - PubMed
-
- Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40: 373–383 - PubMed
-
- Chiara S, Nobile MT, Vincenti M, Lionetto R, Gozza A, Barzacchi MC, Sanguineti O, Repetto L, Rosso R (1998) Advanced colorectal cancer in the elderly: results of consecutive trials with 5-fluorouracil-based chemotherapy. Cancer Chemother Pharmacol 42: 336–340 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical