Altered maturation of peripheral blood dendritic cells in patients with breast cancer
- PMID: 14562018
- PMCID: PMC2394334
- DOI: 10.1038/sj.bjc.6601243
Altered maturation of peripheral blood dendritic cells in patients with breast cancer
Abstract
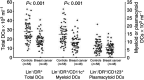
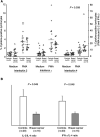
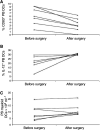
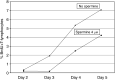
Tumours have at least two mechanisms that can alter dendritic cell (DC) maturation and function. The first affects the ability of haematopoietic progenitors to differentiate into functional DCs; the second affects their differentiation from CD14+ monocytes, promoting an early but dysfunctional maturation. The aim of this study was to evaluate the in vivo relevance of these pathways in breast cancer patients. For this purpose, 53 patients with invasive breast cancer were compared to 68 healthy controls. To avoid isolation or culture procedures for enrichment of DCs, analyses were directly performed by flow cytometry on whole-blood samples. The expression of surface antigens and intracellular accumulation of regulatory cytokines upon LPS stimulation were evaluated. The number of DCs, and in particular of the myeloid subpopulation, was markedly reduced in cancer patients (P<0.001). Patient DCs were characterized by a more mature phenotype compared with controls (P=0.016), and had impaired production of IL-12 (P<0.001). These alterations were reverted by surgical resection of the tumour. To investigate the possible role of some tumour-related immunoactive soluble factors, we measured the plasmatic levels of vascular endothelial growth factor, IL-10 and spermine. A significant inverse correlation between spermine concentration and the percentage of DCs expressing IL-12 was found. Evidence was also obtained that in vitro exposure of monocyte-derived DCs to spermine promoted their activation and maturation, and impaired their function. Taken together, our results suggest that both the above-described mechanisms could concomitantly act in breast cancer to affect DC differentiation, and that spermine could be a mediator of dysfunctional maturation of DCs.
Figures




References
-
- Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, Carbone DP, Gabrilovich DI (2000) Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res 6: 1755–1766 - PubMed
-
- Almeida J, Bueno C, Alguero MC, Sanchez ML, Canizo MC, Fernandez ME, Vaquero JM, Laso FJ, Escribano L, San Miguel JF, Orfao A (1999) Extensive characterization of the immunophenotype and pattern of cytokine production by distinct subpopulations of normal human peripheral blood MHC+/lineage- cells. Clin Exp Immunol 118: 392–401 - PMC - PubMed
-
- Arpinati M, Green CL, Heimfeld S, Heuser JE, Anasetti C (2000) Granulocyte-colony stimulating factor mobilizes T helper 2-inducing dendritic cells. Blood 95: 2484–2490 - PubMed
-
- Bell D, Chomarat P, Broyles D, Netto G, Harb GM, Lebecque S, Valladeau J, Davoust J, Palucka KA, Banchereau J (1999) In breast carcinoma tissue, immature dendritic cells reside within the tumour, whereas mature dendritic cells are located in peritumoral areas. J Exp Med 190: 1417–1425 - PMC - PubMed
-
- Bellone M, Iezzi G, Rovere P, Galati G, Ronchetti A, Protti MP, Davoust J, Rugarli C, Manfredi AA (1997) Processing of engulfed apoptotic bodies yields T cell epitopes. J Immunol 159: 5391–5399 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

