Requirement of the Listeria monocytogenes broad-range phospholipase PC-PLC during infection of human epithelial cells
- PMID: 14563864
- PMCID: PMC219411
- DOI: 10.1128/JB.185.21.6295-6307.2003
Requirement of the Listeria monocytogenes broad-range phospholipase PC-PLC during infection of human epithelial cells
Abstract
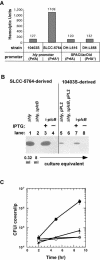
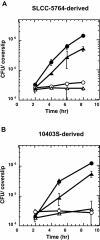
In this study, we investigated the requirement of the Listeria monocytogenes broad-range phospholipase C (PC-PLC) during infection of human epithelial cells. L. monocytogenes is a facultative intracellular bacterial pathogen of humans and a variety of animal species. After entering a host cell, L. monocytogenes is initially surrounded by a membrane-bound vacuole. Bacteria promote their escape from this vacuole, grow within the host cell cytosol, and spread from cell to cell via actin-based motility. Most infection studies with L. monocytogenes have been performed with mouse cells or an in vivo mouse model of infection. In all mouse-derived cells tested, the pore-forming cytolysin listeriolysin O (LLO) is absolutely required for lysis of primary vacuoles formed during host cell entry. However, L. monocytogenes can escape from primary vacuoles in the absence of LLO during infection of human epithelial cell lines Henle 407, HEp-2, and HeLa. Previous studies have shown that the broad-range phospholipase C, PC-PLC, promotes lysis of Henle 407 cell primary vacuoles in the absence of LLO. Here, we have shown that PC-PLC is also required for lysis of HEp-2 and HeLa cell primary vacuoles in the absence of LLO expression. Furthermore, our results indicated that the amount of PC-PLC activity is critical for the efficiency of vacuolar lysis. In an LLO-negative derivative of L. monocytogenes strain 10403S, expression of PC-PLC has to increase before or upon entry into human epithelial cells, compared to expression in broth culture, to allow bacterial escape from primary vacuoles. Using a system for inducible PC-PLC expression in L. monocytogenes, we provide evidence that phospholipase activity can be increased by elevated expression of PC-PLC or Mpl, the enzyme required for proteolytic activation of PC-PLC. Lastly, by using the inducible PC-PLC expression system, we demonstrate that, in the absence of LLO, PC-PLC activity is not only required for lysis of primary vacuoles in human epithelial cells but is also necessary for efficient cell-to-cell spread. We speculate that the additional requirement for PC-PLC activity is for lysis of secondary double-membrane vacuoles formed during cell-to-cell spread.
Figures



References
-
- Bishop, D. K., and D. J. Hinrichs. 1987. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J. Immunol. 139:2005-2009. - PubMed
-
- Bohne, J., H. Kestler, C. Uebele, Z. Sokolovic, and W. Goebel. 1996. Differential regulation of the virulence genes of Listeria monocytogenes by the transcriptional activator PrfA. Mol. Microbiol. 20:1189-1198. - PubMed
-
- Bohne, J., Z. Sokolovic, and W. Goebel. 1994. Transcriptional regulation of prfA and PrfA-regulated virulence genes in Listeria monocytogenes. Mol. Microbiol. 11:1141-1150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

