The small RING finger protein Z drives arenavirus budding: implications for antiviral strategies
- PMID: 14563923
- PMCID: PMC240730
- DOI: 10.1073/pnas.2133782100
The small RING finger protein Z drives arenavirus budding: implications for antiviral strategies
Abstract
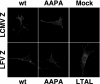
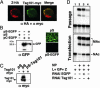
By using a reverse genetics system that is based on the prototypic arenavirus lymphocytic choriomeningitis virus (LCMV), we have identified the arenavirus small RING finger Z protein as the main driving force of virus budding. Both LCMV and Lassa fever virus (LFV) Z proteins exhibited self-budding activity, and both substituted efficiently for the late domain that is present in the Gag protein of Rous sarcoma virus. LCMV and LFV Z proteins contain proline-rich motifs that are characteristic of late domains. Mutations in the PPPY motif of LCMV Z severely impaired the formation of virus-like particles. LFV Z contains two different proline-rich motifs, PPPY and PTAP, which are separated by eight amino acids. Mutational analysis revealed that both motifs are required for efficient LFV Z-mediated budding. Both LCMV and LFV Z proteins recruited to the plasma membrane Tsg101, which is a component of the class E vacuolar protein sorting machinery that has been implicated in budding of HIV and Ebola virus. Targeting of Tsg101 by RNA interference caused a strong reduction in Z-mediated budding. These results indicate that Z is the arenavirus functional counterpart of the matrix proteins found in other negative strand enveloped RNA viruses. Moreover, members of the vacuolar protein sorting pathway appear to play an important role in arena-virus budding. These findings open possibilities for antiviral strategies to combat LFV and other hemorrhagic fever arenaviruses.
Figures
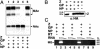


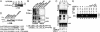
References
-
- Borio, L., Inglesby, T., Peters, C. J., Schmaljohn, A. L., Hughes, J. M., Jahrling, P. B., Ksiazek, T., Johnson, K. M., Meyerhoff, A., O'Toole, T., et al. (2002) J. Am. Med. Assoc. 287 2391-2405. - PubMed
-
- Oldstone, M. B. (2002) in Arenaviruses, ed. Oldstone, M. B. (Springer, Berlin), Vol. 263, pp. 83-118.
-
- Buchmaier, M. J., Bowen, M. D. & Peters, C. J. (2001) in Fields Virology, eds. Knipe, D. M. & Howley, P. M. (Lippincott, Williams & Wilkins, Philadelphia), Vol. 2, pp. 1635-1668.
-
- Cao, W., Henry, M. D., Borrow, P., Yamada, H., Elder, J. H., Ravkov, E. V., Nichol, S. T., Compans, R. W., Campbell, K. P. & Oldstone, M. B. (1998) Science 282 2079-2081. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

