Dissection of the Ascaris sperm motility machinery identifies key proteins involved in major sperm protein-based amoeboid locomotion
- PMID: 14565983
- PMCID: PMC284809
- DOI: 10.1091/mbc.e03-04-0246
Dissection of the Ascaris sperm motility machinery identifies key proteins involved in major sperm protein-based amoeboid locomotion
Abstract
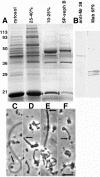
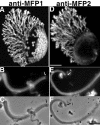
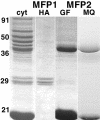
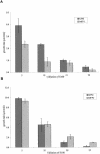
Although Ascaris sperm motility closely resembles that seen in many other types of crawling cells, the lamellipodial dynamics that drive movement result from modulation of a cytoskeleton based on the major sperm protein (MSP) rather than actin. The dynamics of the Ascaris sperm cytoskeleton can be studied in a cell-free in vitro system based on the movement of plasma membrane vesicles by fibers constructed from bundles of MSP filaments. In addition to ATP, MSP, and a plasma membrane protein, reconstitution of MSP motility in this cell-free extract requires cytosolic proteins that orchestrate the site-specific assembly and bundling of MSP filaments that generates locomotion. Here, we identify a fraction of cytosol that is comprised of a small number of proteins but contains all of the soluble components required to assemble fibers. We have purified two of these proteins, designated MSP fiber proteins (MFPs) 1 and 2 and demonstrated by immunolabeling that both are located in the MSP cytoskeleton in cells and in fibers. These proteins had reciprocal effects on fiber assembly in vitro: MFP1 decreased the rate of fiber growth, whereas MFP2 increased the growth rate.
Figures


References
-
- Borisy, G.G., and Svitkina, T.M. (2000). Actin machinery: pushing the envelope. Curr. Opin. Cell Biol. 12, 104-112. - PubMed
-
- Holt, M.R., and Koffer, A. (2001). Cell motility: proline-rich proteins promote protrusions. Trends Cell Biol. 11, 38-46. - PubMed
-
- Italiano, J.E., Jr., Roberts, T.M., Stewart, M., and Fontana, C.A. (1996). Reconstitution in vitro of the motile apparatus from the amoeboid sperm of Ascaris shows that filament assembly and bundling move membranes. Cell 84, 105-114. - PubMed
-
- Italiano, J.E., Jr., Stewart, M., and Roberts, T.M. (2001). How the assembly dynamics of the nematode major sperm protein generate amoeboid cell motility. Int. Rev. Cytol. 202, 1-34. - PubMed
-
- Kay, B.K., Williamson, M.P., and Sudol, M. (2000). The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J. 14, 231-241. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

