Nonlinear elasticity, proteinquakes, and the energy landscapes of functional transitions in proteins
- PMID: 14566052
- PMCID: PMC240658
- DOI: 10.1073/pnas.2135471100
Nonlinear elasticity, proteinquakes, and the energy landscapes of functional transitions in proteins
Abstract
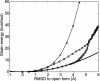
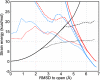
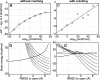
Large-scale motions of biomolecules involve linear elastic deformations along low-frequency normal modes, but for function nonlinearity is essential. In addition, unlike macroscopic machines, biological machines can locally break and then reassemble during function. We present a model for global structural transformations, such as allostery, that involve large-scale motion and possible partial unfolding, illustrating the method with the conformational transition of adenylate kinase. Structural deformation between open and closed states occurs via low-frequency modes on separate reactant and product surfaces, switching from one state to the other when energetically favorable. The switching model is the most straightforward anharmonic interpolation, which allows the barrier for a process to be estimated from a linear normal mode calculation, which by itself cannot be used for activated events. Local unfolding, or cracking, occurs in regions where the elastic stress becomes too high during the transition. Cracking leads to a counterintuitive catalytic effect of added denaturant on allosteric enzyme function. It also leads to unusual relationships between equilibrium constant and rate like those seen recently in single-molecule experiments of motor proteins.
Figures
References
-
- McCammon, J. A., Gelin, B. R., Karplus, M. & Wolynes, P. G. (1976) Nature 262 325–326. - PubMed
-
- Frauenfelder, H., Sligar, S. G. & Wolynes, P. G. (1991) Science 254 1598–1603. - PubMed
-
- Horiuchi, T. & Go, N. (1991) Proteins Struct. Funct. Genet. 10 106–116. - PubMed
-
- Nabarro, F. R. N. (1947) Proc. Phys. Soc. 59 256–272.
-
- Rief, M., Gautel, M., Oesterhelt, F., Fernandez, J. M. & Gaub, H. E. (1997) Science 276 1109–1112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

