Extracellular signal-regulated kinase 2 is necessary for mesoderm differentiation
- PMID: 14566055
- PMCID: PMC240691
- DOI: 10.1073/pnas.2134254100
Extracellular signal-regulated kinase 2 is necessary for mesoderm differentiation
Abstract
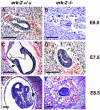
The extracellular signal-regulated kinase (ERK) is a component of the mitogen-activated protein kinase cascade. Exon 2 of erk2 was deleted by homologous recombination and resulted in embryonic lethality at embryonic day 6.5. erk2 mutant embryos did not form mesoderm and showed increased apoptosis but comparable levels of BrdUrd incorporation, indicating a defect in differentiation. erk2 null embryonic stem (ES) cells exhibited reduced total ERK activity upon serum stimulation, augmented ERK1 phosphorylation, and decreased downstream p90Rsk phosphorylation and activity; yet ES cell proliferation was unaffected. Mutant ES cells were capable of forming mesoderm; however, treatment of mutant ES cells with the mitogen-activated protein kinase kinase inhibitor PD184352 decreased total ERK activity and expression of the mesodermal marker brachyury, suggesting that ERK1 can compensate for ERK2 in vitro. Normal embryos at embryonic day 6.5 expressed activated ERK1/2 in the extraembryonic ectoderm, whereas erk2 mutant embryos had no detectable activated ERK1/2 in this region, suggesting that activated ERK1 was not expressed, and therefore cannot compensate for loss of ERK2 in vivo. These data indicate that ERK2 plays an essential role in mesoderm differentiation during embryonic development.
Figures



References
-
- Sugiura, N., Suga, T., Ozeki, Y., Mamiya, G. & Takishima, K. (1997) J. Biol. Chem. 272 21575–21581. - PubMed
-
- Lewis, T. S., Shapiro, P. S. & Ahn, N. G. (1998) Adv. Cancer Res. 74 49–139. - PubMed
-
- Lavoie, J. N., Rivard, N., L'Allemain, G. & Pouyssegur, J. (1996) Prog. Cell Cycle Res. 2, 49–58. - PubMed
-
- Whitmarsh, A. J. & Davis, R. J. (2000) Nature 403 255–256. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

