Elimination in vivo of developing T cells by natural killer cells
- PMID: 14568980
- PMCID: PMC2194238
- DOI: 10.1084/jem.20030918
Elimination in vivo of developing T cells by natural killer cells
Abstract
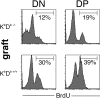
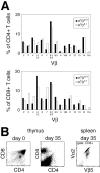
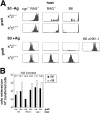
Natural killer cells gauge the absence of self class I MHC on susceptible target cells by means of inhibitory receptors such as members of the Ly49 family. To initiate killing by natural killer cells, a lack of inhibitory signals must be accompanied by the presence of activating ligands on the target cell. Although natural killer cell-mediated rejection of class I MHC-deficient bone marrow (BM) grafts is a matter of record, little is known about the targeting in vivo of specific cellular subsets by natural killer cells. We show here that development of class I MHC-negative thymocytes is delayed as a result of natural killer cell toxicity after grafting of a class I MHC-positive host with class I MHC-negative BM. Double positive thymocytes that persist in the presence of natural killer cells display an unusual T cell receptor-deficient phenotype, yet nevertheless give rise to single positive thymocytes and yield mature class I MHC-deficient lymphocytes that accumulate in the class I MHC-positive host. The resulting class I MHC-deficient CD8 T cells are functional and upon activation remain susceptible to natural killer cell toxicity in vivo. Reconstitution of class I MHC-deficient BM precursors with H2-K(b) by retroviral transduction fully restores normal thymic development.
Figures




Similar articles
-
Positive recognition of MHC class I molecules by the Ly49D receptor of murine NK cells.J Immunol. 1999 Feb 15;162(4):2035-43. J Immunol. 1999. PMID: 9973475
-
T and B lymphocytes exert distinct effects on the homeostasis of NK cells.Eur J Immunol. 2006 Oct;36(10):2725-34. doi: 10.1002/eji.200636011. Eur J Immunol. 2006. PMID: 16955521
-
MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells.Nature. 1992 Jul 2;358(6381):66-70. doi: 10.1038/358066a0. Nature. 1992. PMID: 1614533
-
Allorecognition by murine natural killer cells: lysis of T-lymphoblasts and rejection of bone-marrow grafts.Immunol Rev. 1997 Feb;155:29-40. doi: 10.1111/j.1600-065x.1997.tb00937.x. Immunol Rev. 1997. PMID: 9059880 Review.
-
Regulation of the natural killer cell receptor repertoire.Annu Rev Immunol. 2001;19:291-330. doi: 10.1146/annurev.immunol.19.1.291. Annu Rev Immunol. 2001. PMID: 11244039 Review.
Cited by
-
NKG2D and DNAM-1 activating receptors and their ligands in NK-T cell interactions: role in the NK cell-mediated negative regulation of T cell responses.Front Immunol. 2013 Jan 9;3:408. doi: 10.3389/fimmu.2012.00408. eCollection 2012. Front Immunol. 2013. PMID: 23316196 Free PMC article.
-
NK cells and their ability to modulate T cells during virus infections.Crit Rev Immunol. 2014;34(5):359-88. doi: 10.1615/critrevimmunol.2014010604. Crit Rev Immunol. 2014. PMID: 25404045 Free PMC article. Review.
-
MHC class I limits hippocampal synapse density by inhibiting neuronal insulin receptor signaling.J Neurosci. 2014 Aug 27;34(35):11844-56. doi: 10.1523/JNEUROSCI.4642-12.2014. J Neurosci. 2014. PMID: 25164678 Free PMC article.
-
[Research on the negative immune regulation of NK cells in patients with primary immune thrombocytopenia].Zhonghua Xue Ye Xue Za Zhi. 2017 May 14;38(5):399-403. doi: 10.3760/cma.j.issn.0253-2727.2017.05.009. Zhonghua Xue Ye Xue Za Zhi. 2017. PMID: 28565739 Free PMC article. Chinese.
-
Regulation of activated CD4+ T cells by NK cells via the Qa-1-NKG2A inhibitory pathway.Immunity. 2007 May;26(5):593-604. doi: 10.1016/j.immuni.2007.03.017. Immunity. 2007. PMID: 17509909 Free PMC article.
References
-
- Yu, Y.Y., T. George, J.R. Dorfman, J. Roland, V. Kumar, and M. Bennett. 1996. The role of Ly49A and 5E6(Ly49C) molecules in hybrid resistance mediated by murine natural killer cells against normal T cell blasts. Immunity. 4:67–76. - PubMed
-
- Bix, M., N.S. Liao, M. Zijlstra, J. Loring, R. Jaenisch, and D. Raulet. 1991. Rejection of class I MHC-deficient haemopoietic cells by irradiated MHC-matched mice. Nature. 349:329–331. - PubMed
-
- Lanier, L.L. 1998. NK cell receptors. Annu. Rev. Immunol. 16:359–393. - PubMed
-
- Yokoyama, W.M. 1995. Natural killer cell receptors. Curr. Opin. Immunol. 7:110–120. - PubMed
-
- Yokoyama, W.M. 1993. Recognition structures on natural killer cells. Curr. Opin. Immunol. 5:67–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

