Herpes simplex virus triggers activation of calcium-signaling pathways
- PMID: 14568989
- PMCID: PMC2173509
- DOI: 10.1083/jcb.200301084
Herpes simplex virus triggers activation of calcium-signaling pathways
Abstract
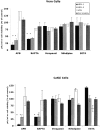
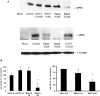
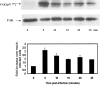
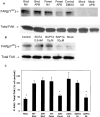
The cellular pathways required for herpes simplex virus (HSV) invasion have not been defined. To test the hypothesis that HSV entry triggers activation of Ca2+-signaling pathways, the effects on intracellular calcium concentration ([Ca2+]i) after exposure of cells to HSV were examined. Exposure to virus results in a rapid and transient increase in [Ca2+]i. Pretreatment of cells with pharmacological agents that block release of inositol 1,4,5-triphosphate (IP3)-sensitive endoplasmic reticulum stores abrogates the response. Moreover, treatment of cells with these pharmacological agents inhibits HSV infection and prevents focal adhesion kinase (FAK) phosphorylation, which occurs within 5 min after viral infection. Viruses deleted in glycoprotein L or glycoprotein D, which bind but do not penetrate, fail to induce a [Ca2+]i response or trigger FAK phosphorylation. Together, these results support a model for HSV infection that requires activation of IP3-responsive Ca2+-signaling pathways and that is associated with FAK phosphorylation. Defining the pathway of viral invasion may lead to new targets for anti-viral therapy.
Figures








References
-
- Baldwin, B.R., C.O. Zhang, and S. Keay. 2000. Cloning and epitope mapping of a functional partial fusion receptor for human cytomegalovirus gH. J. Gen. Virol. 81:27–35. - PubMed
-
- Bierne, H., S. Dramsi, M.P. Gratacap, C. Randriamampita, G. Carpenter, B. Payrastre, and P. Cossart. 2000. The invasion protein InIB from Listeria monocytogenes activates PLC-gamma1 downstream from PI 3-kinase. Cell. Microbiol. 2:465–476. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

