The Tat/TAR-dependent phosphorylation of RNA polymerase II C-terminal domain stimulates cotranscriptional capping of HIV-1 mRNA
- PMID: 14569024
- PMCID: PMC240675
- DOI: 10.1073/pnas.1835726100
The Tat/TAR-dependent phosphorylation of RNA polymerase II C-terminal domain stimulates cotranscriptional capping of HIV-1 mRNA
Abstract
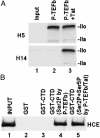
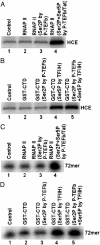
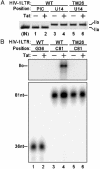
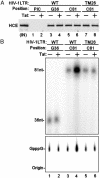
The HIV type 1 (HIV-1) Tat protein stimulates transcription elongation by recruiting P-TEFb (CDK9/cyclin T1) to the transactivation response (TAR) RNA structure. Tat-induced CDK9 kinase has been shown to phosphorylate Ser-5 of RNA polymerase II (RNAP II) C-terminal domain (CTD). Results presented here demonstrate that Tat-induced Ser-5 phosphorylation of CTD by P-TEFb stimulates the guanylyltransferase activity of human capping enzyme and RNA cap formation. Sequential phosphorylation of CTD by Tat-induced P-TEFb enhances the stimulation of human capping enzyme guanylyltransferase activity and RNA cap formation by transcription factor IIH-mediated CTD phosphorylation. Using an immobilized template assay that permits isolation of transcription complexes, we show that Tat/TAR-dependent phosphorylation of RNAP II CTD stimulates cotranscriptional capping of HIV-1 mRNA. Upon transcriptional induction of latently infected cells, accumulation of capped transcripts occurs along with Ser-5-phosphorylated RNAP II in the promoter proximal region of the HIV-1 genome. Therefore, these observations suggest that Tat/TAR-dependent phosphorylation of RNAP II CTD is crucial not only in promoting transcription elongation but also in stimulating nascent viral RNA capping.
Figures


References
-
- Shuman, S. (2001) Prog. Nucleic Acid Res. Mol. Biol. 66 1–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

