The role of microtubule-associated protein 2c in the reorganization of microtubules and lamellipodia during neurite initiation
- PMID: 14573527
- PMCID: PMC6740480
- DOI: 10.1523/JNEUROSCI.23-29-09479.2003
The role of microtubule-associated protein 2c in the reorganization of microtubules and lamellipodia during neurite initiation
Abstract
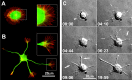
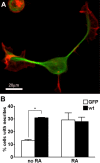
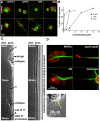
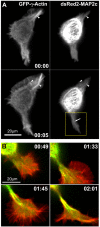
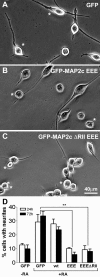
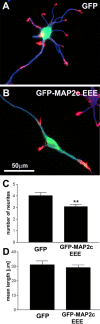
During neurite initiation, cells surrounded by a flattened, actin-rich lamellipodium transform to produce thin, microtubule-filled neurite shafts tipped by actin-rich growth cones, but little is known about this transformation. Our detailed time-lapse analyses of cultured hippocampal neurons, a widely used model system for neuronal development, revealed that neurites emerge from segmented lamellipodia, which then gradually extend from the cell body to become nascent growth cones. This suggests that actin- and microtubule-rich structures are reorganized in a coordinated manner. We hypothesized that proteins such as microtubule-associated protein 2 (MAP2), which can interact with both cytoskeletal components, might be critically involved in neurite initiation. Live-cell video and fluorescence microscopy in Neuro-2a cells showed that expression of MAP2c triggers neurite formation via rapid accumulation and bundling of stable, MAP2c-bound microtubules, concurrent with a gradual transformation of lamellipodia into nascent growth cones. The microtubule-stabilizing agent Taxol did not mimic this effect, suggesting that the ability of MAP2c to stabilize microtubules is not sufficient for neurite initiation. However, combination of Taxol treatment with actin disruption induced robust process formation, suggesting that inhibitory effects of F-actin need to be overcome as well. Neurite initiation by MAP2c required its microtubule-binding domain and was enhanced by its binding domain for cAMP-dependent protein kinase (PKA). MAP2c mutants defective in both PKA and microtubule binding acted as dominant negative inhibitors of neurite initiation in neuroblastoma cells and primary hippocampal neurons. Together, these data suggest that MAP2c bears functions that both stabilize microtubules and directly or indirectly alter actin organization during neurite initiation.
Figures



References
-
- Bernhardt R, Matus A ( 1984) Light and electron microscopic studies of the distribution of microtubule-associated protein 2 in rat brain: a difference between dendritic and axonal cytoskeleton. J Comp Neurol 226: 203-221. - PubMed
-
- Bubb MR, Spector I, Beyer BB, Fosen KM ( 2000) Effects of jasplakinolide on the kinetics of actin polymerization: an explanation for certain in vivo observations. J Biol Chem 275: 5163-5170. - PubMed
-
- Caceres A, Mautino J, Kosik KS ( 1992) Suppression of MAP2 in cultured cerebellar macroneurons inhibits minor neurite formation. Neuron 9: 607-618. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
