Firing properties of spinal interneurons during voluntary movement. I. State-dependent regularity of firing
- PMID: 14573540
- PMCID: PMC6740472
- DOI: 10.1523/JNEUROSCI.23-29-09600.2003
Firing properties of spinal interneurons during voluntary movement. I. State-dependent regularity of firing
Abstract
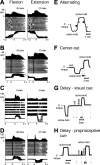
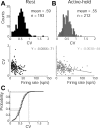
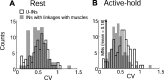
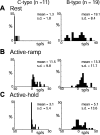
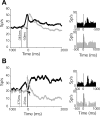
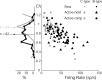
The firing properties of single spinal interneurons (INs) were studied in five awake, behaving monkeys performing isometric or auxotonic flexion-extension torques at the wrist. INs tended to fire tonically at rest (mean rate, 14 spikes (sp)/sec) and during generation of static torque (mean rate, 19 sp/sec in flexion, 24 sp/sec in extension). INs exhibited regular firing, with autocorrelation functions showing clear periodic features and a mean coefficient of variation of interspike intervals (CV) of 0.55 during production of static torque. For the population, there was an inverse correlation between CV and mean rate. However, 46% of the INs had task-dependent changes in regularity that were not predicted by changes in firing rate, suggesting that their firing pattern is determined not only by the intrinsic properties of the neurons but also by the properties of its synaptic inputs. INs showed two main response types to passive wrist displacement: biphasic and coactivation. Cells with these sensory responses had different, stereotypical temporal activity profiles and firing regularity during active movement. However, INs having correlational linkages with forearm muscles, identified as features in spike-triggered averages of electromyographic activity, did not exhibit unique responses or firing properties, although they tended to fire more regularly than other INs. This suggests the lack of a precise mapping of inputs to outputs for the spinal premotor network.
Figures





References
-
- Abeles M, Lass Y ( 1975) Transmission of information by the axon. II. The channel capacity. Biol Cybern 19: 121-125. - PubMed
-
- Baker SN, Spinks R, Jackson A, Lemon RN ( 2001) Synchronization in monkey motor cortex during a precision grip task. I. Task-dependent modulation in single-unit synchrony. J Neurophysiol 85: 869-885. - PubMed
-
- Beierholm U, Nielsen CD, Ryge J, Alstrom P, Kiehn O ( 2001) Characterization of reliability of spike timing in spinal interneurons during oscillating inputs. J Neurophysiol 86: 1858-1868. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
