Major differences in inhibitory synaptic transmission onto two neocortical interneuron subclasses
- PMID: 14573546
- PMCID: PMC6740477
- DOI: 10.1523/JNEUROSCI.23-29-09664.2003
Major differences in inhibitory synaptic transmission onto two neocortical interneuron subclasses
Abstract
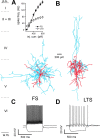
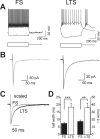
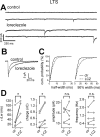
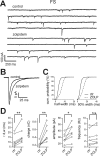
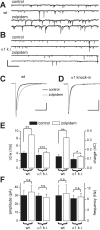
Locally projecting GABAergic interneurons are the major providers of inhibition in the neocortex and play a crucial role in several brain functions. Neocortical interneurons are connected via electrical and chemical synapses that may be crucial in modulating complex network oscillations. We investigated the properties of spontaneous and evoked IPSCs in two morphologically and physiologically identified interneuron subtypes, the fast-spiking (FS) and low threshold-spiking (LTS) cells in layer V of rodent sensorimotor cortex. We found that IPSCs recorded in FS cells were several orders of magnitude more frequent, larger in amplitude, and had faster kinetics than IPSCs recorded in LTS cells. GABA(A) receptor alpha- and beta-subunit selective modulators, zolpidem and loreclezole, had different effects on IPSCs in FS and LTS interneurons, suggesting differential expression of GABA(A) receptor subunit subtypes. These pharmacological data indicated that the alpha1 subunit subtype is poorly expressed by LTS cells but makes a large contribution to GABA(A) receptors on FS cells. This was confirmed by experiments performed in genetically modified mice in which the alpha1 subunit had been made insensitive to benzodiazepine-like agonists. These results suggest that differences in IPSC waveform are likely attributable to distinctive expression of GABA(A) receptor subunits in FS and LTS cells. The particular properties of GABAergic input on different interneuronal subtypes might have important consequences for generation and pacing of cortical rhythms underlying several brain functions. Moreover, selective pharmacological manipulation of distinct inhibitory circuits might allow regulation of pyramidal cell activities under specific physiological and pathophysiological conditions.
Figures




References
-
- Banks MI, Hardie JB, Pearce RA ( 2002) Development of GABA(A) receptor-mediated inhibitory postsynaptic currents in hippocampus. J Neurophysiol 88: 3097-3107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
