Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons
- PMID: 14573548
- PMCID: PMC6740462
- DOI: 10.1523/JNEUROSCI.23-29-09687.2003
Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons
Abstract
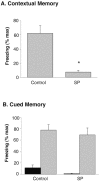
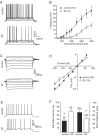
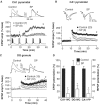
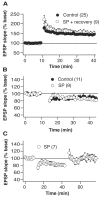
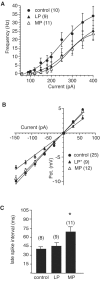
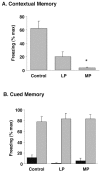
Although the function of sleep remains elusive, several lines of evidence suggest that sleep has an important role in learning and memory. In light of the available data and with the prevalence of sleep deprivation (SD), we sought to determine the effect of SD on neuronal functioning. We found that the exposure of rats to 72 hr of primarily rapid eye movement SD impaired their subsequent performance on a hippocampus-dependent spatial learning task but had no effect on an amygdala-dependent learning task. To determine the underlying cellular level mechanisms of this hippocampal deficit, we examined the impact of SD on several fundamental aspects of membrane excitability and synaptic physiology in hippocampal CA1 pyramidal neurons and dentate gyrus granule cells. We found that neuronal excitability was severely reduced in CA1 neurons but not in granule cells and that the production of long-term potentiation of synaptic strength was inhibited in both areas. Using multiple SD methods we further attempted to differentiate the effects of sleep deprivation from those associated with the nonspecific stress induced by the sleep deprivation methods. Together these data suggest that failure to acquire adequate sleep produces several molecular and cellular level alterations that profoundly inhibit hippocampal functioning.
Figures

References
-
- Bliss TVP, Collingridge GL ( 1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361: 31-39. - PubMed
-
- Bramham CR, Southard T, Ahlers ST, Sarvey JM ( 1998) Acute cold stress leading to elevated corticosterone neither enhances synaptic efficacy nor impairs LTP in the dentate gyrus of freely moving rats. Brain Res 789: 245-255. - PubMed
-
- Buzsaki G ( 1998) Memory consolidation during sleep: a neurophysiological perspective. J Sleep Res 7: 17-23. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
