Ribonucleotide reduction in Mycobacterium tuberculosis: function and expression of genes encoding class Ib and class II ribonucleotide reductases
- PMID: 14573627
- PMCID: PMC219568
- DOI: 10.1128/IAI.71.11.6124-6131.2003
Ribonucleotide reduction in Mycobacterium tuberculosis: function and expression of genes encoding class Ib and class II ribonucleotide reductases
Abstract
Mycobacterium tuberculosis, the causative agent of tuberculosis, possesses a class Ib ribonucleotide reductase (RNR), encoded by the nrdE and nrdF2 genes, in addition to a putative class II RNR, encoded by nrdZ. In this study we probed the relative contributions of these RNRs to the growth and persistence of M. tuberculosis. We found that targeted knockout of the nrdF2 gene could be achieved only in the presence of a complementing allele, confirming that this gene is essential under normal, in vitro growth conditions. This observation also implied that the alternate class Ib small subunit encoded by the nrdF1 gene is unable to substitute for nrdF2 and that the class II RNR, NrdZ, cannot substitute for the class Ib enzyme, NrdEF2. Conversely, a DeltanrdZ null mutant of M. tuberculosis was readily obtained by allelic exchange mutagenesis. Quantification of levels of nrdE, nrdF2, nrdF1, and nrdZ gene expression by real-time, quantitative reverse transcription-PCR with molecular beacons by using mRNA from aerobic and O(2)-limited cultures showed that nrdZ was significantly induced under microaerophilic conditions, in contrast to the other genes, whose expression was reduced by O(2) restriction. However, survival of the DeltanrdZ mutant strain was not impaired under hypoxic conditions in vitro. Moreover, the lungs of B6D2/F(1) mice infected with the DeltanrdZ mutant had bacterial loads comparable to those of lungs infected with the parental wild-type strain, which argues against the hypothesis that nrdZ plays a significant role in the virulence of M. tuberculosis in this mouse model.
Figures

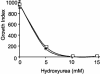


References
-
- Blondelet-Rouault, M. H., J. Weiser, A. Lebrihi, P. Branny, and J. L. Pernodet. 1997. Antibiotic resistance gene cassettes derived from the omega interposon for use in E. coli and Streptomyces. Gene 190:315-317. - PubMed
-
- Bloom, B. R., and P. M. Small. 1998. The evolving relation between humans and Mycobacterium tuberculosis. N. Engl. J. Med. 338:677-678. - PubMed
-
- Booker, S., S. Licht, J. Broderick, and J. Stubbe. 1994. Coenzyme B12-dependent ribonucleotide reductase: evidence for the participation of five cysteine residues in ribonucleotide reduction. Biochemistry 33:12676-12685. - PubMed
-
- Borovok, I., R. Kreisberg-Zakarin, M. Yanko, R. Schreiber, M. Myslovati, F. Aslund, A. Holmgren, G. Cohen, and Y. Aharonowitz. 2002. Streptomyces spp. contain class Ia and class II ribonucleotide reductases: expression analysis of the genes in vegetative growth. Microbiology 148:391-404. - PubMed
-
- Boshoff, H. I. M., M. B. Reed, C. E. Barry III, and V. Mizrahi. 2003. DnaE2 polymerase contributes to in vivo survival and the emergence of drug resistance in Mycobacterium tuberculosis. Cell 113:183-193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

