Phage lytic enzyme Cpl-1 as a novel antimicrobial for pneumococcal bacteremia
- PMID: 14573637
- PMCID: PMC219578
- DOI: 10.1128/IAI.71.11.6199-6204.2003
Phage lytic enzyme Cpl-1 as a novel antimicrobial for pneumococcal bacteremia
Abstract
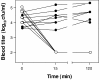
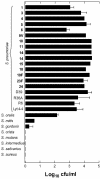
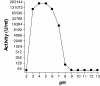
Streptococcus pneumoniae is becoming increasingly antibiotic resistant worldwide, and thus new antimicrobials are badly needed. We report the use of Cpl-1, the lytic enzyme of a pneumococcal bacteriophage, as an intravenous therapy for pneumococcal bacteremia in a mouse model. A 2000- microg dose of Cpl-1 reduced pneumococcal titers from a median of log(10) 4.70 CFU/ml to undetectable levels (<log(10) 2.00 CFU/ml) within 15 min. This dose given 1 h after intravenous infection led to 100% survival at 48 h, compared to the 20% survival of buffer-treated controls. In advanced bacteremia, treatment with two doses at 5 and 10 h still resulted in significantly longer survival (P < 0.0001) and a hazard ratio of 0.29 (95% confidence interval, 0.04 to 0.35). The enzyme is immunogenic, but the treatment efficacy was not significantly diminished after previous intravenous exposure of mice and hyperimmune rabbit serum did not neutralize the activity. Cpl-1 is also very effective as a topical nasal treatment against colonization by S. pneumoniae. In vitro, the enzyme is active against many serotypes of S. pneumoniae, independent of their penicillin resistance, and it is very specific for this species. Bacteriophage enzymes are unusual but extremely effective antimicrobials and represent a new weapon against infections with resistant bacteria.
Figures


Similar articles
-
A novel chimeric phage lysin with high in vitro and in vivo bactericidal activity against Streptococcus pneumoniae.J Antimicrob Chemother. 2015;70(6):1763-73. doi: 10.1093/jac/dkv038. Epub 2015 Mar 1. J Antimicrob Chemother. 2015. PMID: 25733585
-
Phage lytic enzymes as therapy for antibiotic-resistant Streptococcus pneumoniae infection in a murine sepsis model.J Antimicrob Chemother. 2003 Dec;52(6):967-73. doi: 10.1093/jac/dkg485. Epub 2003 Nov 12. J Antimicrob Chemother. 2003. PMID: 14613958
-
Bactericidal synergism between daptomycin and the phage lysin Cpl-1 in a mouse model of pneumococcal bacteraemia.Int J Antimicrob Agents. 2013 Nov;42(5):416-21. doi: 10.1016/j.ijantimicag.2013.06.020. Epub 2013 Aug 9. Int J Antimicrob Agents. 2013. PMID: 23992647
-
Pneumococcal infection in children: rational antibiotic choice for drug-resistant Streptococcus pneumoniae.Acta Paediatr Taiwan. 2003 Mar-Apr;44(2):67-74. Acta Paediatr Taiwan. 2003. PMID: 12845845 Review.
-
The importance of pharmacodynamic properties in treatment of penicillin resistant Streptococcus pneumoniae.Dan Med Bull. 2000 Nov;47(5):313-27. Dan Med Bull. 2000. PMID: 11155659 Review.
Cited by
-
In vitro destruction of Streptococcus pneumoniae biofilms with bacterial and phage peptidoglycan hydrolases.Antimicrob Agents Chemother. 2011 Sep;55(9):4144-8. doi: 10.1128/AAC.00492-11. Epub 2011 Jul 11. Antimicrob Agents Chemother. 2011. PMID: 21746941 Free PMC article.
-
Bacteriophage lysins as effective antibacterials.Curr Opin Microbiol. 2008 Oct;11(5):393-400. doi: 10.1016/j.mib.2008.09.012. Epub 2008 Oct 14. Curr Opin Microbiol. 2008. PMID: 18824123 Free PMC article. Review.
-
Bacteriophage endolysins as a potential weapon to combat Clostridioides difficile infection.Gut Microbes. 2020 Nov 9;12(1):1813533. doi: 10.1080/19490976.2020.1813533. Gut Microbes. 2020. PMID: 32985336 Free PMC article. Review.
-
Isolation and expression of the lysis genes of Actinomyces naeslundii phage Av-1.Appl Environ Microbiol. 2006 Feb;72(2):1110-7. doi: 10.1128/AEM.72.2.1110-1117.2006. Appl Environ Microbiol. 2006. PMID: 16461656 Free PMC article.
-
Linker Editing of Pneumococcal Lysin ClyJ Conveys Improved Bactericidal Activity.Antimicrob Agents Chemother. 2020 Jan 27;64(2):e01610-19. doi: 10.1128/AAC.01610-19. Print 2020 Jan 27. Antimicrob Agents Chemother. 2020. PMID: 31767724 Free PMC article.
References
-
- Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. R. Hansen, L. Elvin, K. M. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, and K. Edwards. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr. Infect. Dis. J. 19:187-195. - PubMed
-
- Black, S. B., H. R. Shinefield, S. Ling, J. Hansen, B. Fireman, D. Spring, J. Noyes, E. Lewis, P. Ray, J. Lee, and J. Hackell. 2002. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr. Infect. Dis. J. 21:810-815. - PubMed
-
- Brucato, F. H., and S. V. Pizzo. 1990. Catabolism of streptokinase and polyethylene glycol-streptokinase: evidence for transport of intact forms through the biliary system in the mouse. Blood 76:73-79. - PubMed
-
- Dagan, R., N. Givon-Lavi, O. Zamir, M. Sikuler-Cohen, L. Guy, J. Janco, P. Yagupsky, and D. Fraser. 2002. Reduction of nasopharyngeal carriage of Streptococcus pneumoniae after administration of a 9-valent pneumococcal conjugate vaccine to toddlers attending day care centers. J. Infect. Dis. 185:927-936. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

