Phage lytic enzyme Cpl-1 as a novel antimicrobial for pneumococcal bacteremia
- PMID: 14573637
- PMCID: PMC219578
- DOI: 10.1128/IAI.71.11.6199-6204.2003
Phage lytic enzyme Cpl-1 as a novel antimicrobial for pneumococcal bacteremia
Abstract
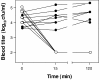
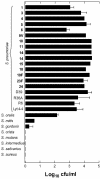
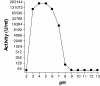
Streptococcus pneumoniae is becoming increasingly antibiotic resistant worldwide, and thus new antimicrobials are badly needed. We report the use of Cpl-1, the lytic enzyme of a pneumococcal bacteriophage, as an intravenous therapy for pneumococcal bacteremia in a mouse model. A 2000- microg dose of Cpl-1 reduced pneumococcal titers from a median of log(10) 4.70 CFU/ml to undetectable levels (<log(10) 2.00 CFU/ml) within 15 min. This dose given 1 h after intravenous infection led to 100% survival at 48 h, compared to the 20% survival of buffer-treated controls. In advanced bacteremia, treatment with two doses at 5 and 10 h still resulted in significantly longer survival (P < 0.0001) and a hazard ratio of 0.29 (95% confidence interval, 0.04 to 0.35). The enzyme is immunogenic, but the treatment efficacy was not significantly diminished after previous intravenous exposure of mice and hyperimmune rabbit serum did not neutralize the activity. Cpl-1 is also very effective as a topical nasal treatment against colonization by S. pneumoniae. In vitro, the enzyme is active against many serotypes of S. pneumoniae, independent of their penicillin resistance, and it is very specific for this species. Bacteriophage enzymes are unusual but extremely effective antimicrobials and represent a new weapon against infections with resistant bacteria.
Figures


References
-
- Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. R. Hansen, L. Elvin, K. M. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, and K. Edwards. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr. Infect. Dis. J. 19:187-195. - PubMed
-
- Black, S. B., H. R. Shinefield, S. Ling, J. Hansen, B. Fireman, D. Spring, J. Noyes, E. Lewis, P. Ray, J. Lee, and J. Hackell. 2002. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr. Infect. Dis. J. 21:810-815. - PubMed
-
- Brucato, F. H., and S. V. Pizzo. 1990. Catabolism of streptokinase and polyethylene glycol-streptokinase: evidence for transport of intact forms through the biliary system in the mouse. Blood 76:73-79. - PubMed
-
- Dagan, R., N. Givon-Lavi, O. Zamir, M. Sikuler-Cohen, L. Guy, J. Janco, P. Yagupsky, and D. Fraser. 2002. Reduction of nasopharyngeal carriage of Streptococcus pneumoniae after administration of a 9-valent pneumococcal conjugate vaccine to toddlers attending day care centers. J. Infect. Dis. 185:927-936. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

