Pathogenic Acanthamoeba spp secrete a mannose-induced cytolytic protein that correlates with the ability to cause disease
- PMID: 14573643
- PMCID: PMC219557
- DOI: 10.1128/IAI.71.11.6243-6255.2003
Pathogenic Acanthamoeba spp secrete a mannose-induced cytolytic protein that correlates with the ability to cause disease
Abstract
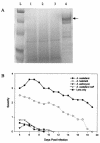
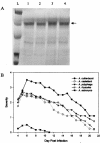
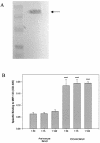
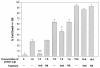
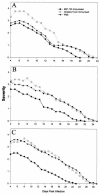
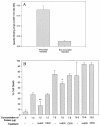
The pathogenesis of Acanthamoeba keratitis begins when Acanthamoeba trophozoites bind specifically to mannosylated glycoproteins upregulated on the surfaces of traumatized corneal epithelial cells. When Acanthamoeba castellanii trophozoites are grown in methyl-alpha-D-mannopyranoside, they are induced to secrete a novel 133-kDa protein that is cytolytic to corneal epithelial cells. Clinical isolates of Acanthamoeba spp., and not the soil isolates, were proficient at producing a mannose-induced protein (MIP-133) and generating disease in Chinese hamsters. The purified protein was efficient at killing corneal epithelial cells, the first mechanistic barrier, by inducing apoptosis in a caspase 3-dependent pathway. Subsequent steps in pathogenesis require the amoebae to penetrate and degrade collagen. Only the clinical isolates tested were efficient at migrating through a collagenous matrix in vitro, presumably by MIP-133 degradation of both human type I and human type IV collagen. A chicken anti-MIP-133 antiserum effectively bound to the protein and blocked collagenolytic activity, migration, and cytopathic effects (CPE) against corneal cells in vitro. Chinese hamsters orally immunized with MIP-133 displayed a >30% reduction in disease. Immunoglobulin A isolated from immunized animals bound MIP-133 and blocked CPE on corneal cells in vitro. Animals induced to generate severe chronic infections displayed significant reductions in disease symptoms upon oral immunization postinfection. These data suggest that MIP-133 production might be necessary to initiate corneal disease and that it may play an important role in the subsequent steps of the pathogenic cascade of Acanthamoeba keratitis. Furthermore, as antibodies produced both prior to and after infection reduced clinical symptoms of disease, the protein may represent an important immunotherapeutic target for Acanthamoeba keratitis.
Figures




Similar articles
-
Role of phospholipase A₂ (PLA₂) inhibitors in attenuating apoptosis of the corneal epithelial cells and mitigation of Acanthamoeba keratitis.Exp Eye Res. 2013 Aug;113:182-91. doi: 10.1016/j.exer.2013.05.021. Epub 2013 Jun 20. Exp Eye Res. 2013. PMID: 23792108 Free PMC article.
-
Effect of immunization with the mannose-induced Acanthamoeba protein and Acanthamoeba plasminogen activator in mitigating Acanthamoeba keratitis.Invest Ophthalmol Vis Sci. 2007 Dec;48(12):5597-604. doi: 10.1167/iovs.07-0407. Invest Ophthalmol Vis Sci. 2007. PMID: 18055809
-
Effects of mannose on Acanthamoeba castellanii proliferation and cytolytic ability to corneal epithelial cells.Invest Ophthalmol Vis Sci. 2003 Aug;44(8):3424-31. doi: 10.1167/iovs.03-0019. Invest Ophthalmol Vis Sci. 2003. PMID: 12882791
-
Pathogenesis of acanthamoeba keratitis.Ocul Surf. 2010 Apr;8(2):70-9. doi: 10.1016/s1542-0124(12)70071-x. Ocul Surf. 2010. PMID: 20427010 Free PMC article. Review.
-
The role of the innate and adaptive immune responses in Acanthamoeba keratitis.Arch Immunol Ther Exp (Warsz). 2002;50(1):53-9. Arch Immunol Ther Exp (Warsz). 2002. PMID: 11916309 Review.
Cited by
-
Proteases of Acanthamoeba.Parasitol Res. 2023 Dec 8;123(1):19. doi: 10.1007/s00436-023-08059-z. Parasitol Res. 2023. PMID: 38063887 Review.
-
Proteases from Entamoeba spp. and Pathogenic Free-Living Amoebae as Virulence Factors.J Trop Med. 2013;2013:890603. doi: 10.1155/2013/890603. Epub 2013 Feb 7. J Trop Med. 2013. PMID: 23476670 Free PMC article.
-
Role of protease-activated receptors 2 (PAR2) in ocular infections and inflammation.Receptors Clin Investig. 2014;1(6):e2991. doi: 10.14800/rci.291. Receptors Clin Investig. 2014. PMID: 26078987 Free PMC article.
-
Pathobiology and Immunobiology of Acanthamoeba Keratitis: Insights from Animal Models .Yale J Biol Med. 2017 Jun 23;90(2):261-268. eCollection 2017 Jun. Yale J Biol Med. 2017. PMID: 28656012 Free PMC article. Review.
-
Role of phospholipase A₂ (PLA₂) inhibitors in attenuating apoptosis of the corneal epithelial cells and mitigation of Acanthamoeba keratitis.Exp Eye Res. 2013 Aug;113:182-91. doi: 10.1016/j.exer.2013.05.021. Epub 2013 Jun 20. Exp Eye Res. 2013. PMID: 23792108 Free PMC article.
References
-
- Alizadeh, H., S. Apte, M. S. El-Agha, L. Li, M. Hurt, K. Howard, H. D. Cavanagh, J. P. McCulley, and J. Y. Niederkorn. 2001. Tear IgA and serum IgG antibodies against Acanthamoeba in patients with Acanthamoeba keratitis. Cornea 20:622-627. - PubMed
-
- Alizadeh, H., J. Y. Niederkorn, and J. P. McCulley. 1996. Acanthamoeba keratitis, p. 1062-1071. In J. S. Pepose, G. N. Holland, and K. R. Wilhelmus (ed.), Ocular infection and immunity. Mosby, St. Louis, Mo.
-
- Auran, J. D., M. B. Starr, and F. A. Jacobiec. 1987. Acanthamoeba keratitis. Cornea 6:2-26. - PubMed
-
- Centers for Disease Control. 1987. Acanthamoeba keratitis in soft-contact-lens wearers—United States. Morb. Mortal. Wkly. Rep. 36:397-398, 403-404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

