Transfer region of pO113 from enterohemorrhagic Escherichia coli: similarity with R64 and identification of a novel plasmid-encoded autotransporter, EpeA
- PMID: 14573650
- PMCID: PMC219559
- DOI: 10.1128/IAI.71.11.6307-6319.2003
Transfer region of pO113 from enterohemorrhagic Escherichia coli: similarity with R64 and identification of a novel plasmid-encoded autotransporter, EpeA
Abstract
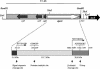
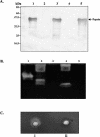
Enterohemorrhagic Escherichia coli (EHEC) is a prominent, food-borne cause of diarrhea, bloody diarrhea, and the hemolytic uremic syndrome in industrialized countries. Most strains of EHEC carry the locus for enterocyte effacement (LEE) pathogenicity island, but a proportion of isolates from patients with severe disease do not carry LEE and very little is known about virulence factors in these organisms. LEE-negative strains of EHEC typically express Shiga toxin 2 and carry a large plasmid that encodes the production of EHEC hemolysin. In this study, we determined the nucleotide sequence of the transfer region of pO113, the large hemolysin plasmid from LEE-negative EHEC O113:H21 (EH41). This 63.9-kb region showed a high degree of similarity with the transfer region of R64, and pO113 was capable of self-transmission at low frequencies. Unlike R64 and the related dot/icm system of Legionella pneumophila, however, pO113 was unable to mobilize RSF1010. In addition, the pO113 transfer region encoded a novel high-molecular-weight serine protease autotransporter of Enterobacteriaceae (SPATE) protein, termed EpeA. Like other SPATEs, EpeA exhibited protease activity and mucinase activity, but expression was not associated with a cytopathic effect on epithelial cells. Analysis of a second high-molecular-weight secreted protein revealed that pO113 also encodes EspP, a cytopathic SPATE identified previously in EHEC O157:H7. The nucleotide sequences encoding the predicted beta-domains of espP and epeA were identical and also shared significant homology with a third SPATE protein, EspI. Both espP and epeA were detected in several LEE-negative clinical isolates of EHEC and thus may contribute to the pathogenesis of this subset of EHEC.
Figures





Similar articles
-
Identification of a novel fimbrial gene cluster related to long polar fimbriae in locus of enterocyte effacement-negative strains of enterohemorrhagic Escherichia coli.Infect Immun. 2002 Dec;70(12):6761-9. doi: 10.1128/IAI.70.12.6761-6769.2002. Infect Immun. 2002. PMID: 12438351 Free PMC article.
-
Evolution of enterohemorrhagic Escherichia coli hemolysin plasmids and the locus for enterocyte effacement in shiga toxin-producing E. coli.Infect Immun. 1998 Jun;66(6):2553-61. doi: 10.1128/IAI.66.6.2553-2561.1998. Infect Immun. 1998. PMID: 9596716 Free PMC article.
-
Shiga toxin-producing Escherichia coli strains negative for locus of enterocyte effacement.Emerg Infect Dis. 2009 Mar;15(3):372-80. doi: 10.3201/eid1503.080631. Emerg Infect Dis. 2009. PMID: 19239748 Free PMC article.
-
[Genes involved in the virulence of enterohemorrhagic Escherichia coli].Nihon Rinsho. 1997 Mar;55(3):641-5. Nihon Rinsho. 1997. PMID: 9086773 Review. Japanese.
-
Interkingdom Chemical Signaling in Enterohemorrhagic Escherichia coli O157:H7.Adv Exp Med Biol. 2016;874:201-13. doi: 10.1007/978-3-319-20215-0_9. Adv Exp Med Biol. 2016. PMID: 26589220 Review.
Cited by
-
CTX-M-15 extended-spectrum β-lactamase in a shiga toxin-producing Escherichia coli isolate of serotype O111:H8.Appl Environ Microbiol. 2012 Feb;78(4):1308-9. doi: 10.1128/AEM.06997-11. Epub 2011 Dec 9. Appl Environ Microbiol. 2012. PMID: 22156432 Free PMC article.
-
Distribution of the serine protease autotransporters of the Enterobacteriaceae among extraintestinal clinical isolates of Escherichia coli.J Clin Microbiol. 2005 Aug;43(8):4076-82. doi: 10.1128/JCM.43.8.4076-4082.2005. J Clin Microbiol. 2005. PMID: 16081954 Free PMC article.
-
Phylogenetic Classification and Functional Review of Autotransporters.Front Immunol. 2022 Jul 1;13:921272. doi: 10.3389/fimmu.2022.921272. eCollection 2022. Front Immunol. 2022. PMID: 35860281 Free PMC article. Review.
-
Serine protease autotransporters from Shigella flexneri and pathogenic Escherichia coli target a broad range of leukocyte glycoproteins.Proc Natl Acad Sci U S A. 2011 Aug 2;108(31):12881-6. doi: 10.1073/pnas.1101006108. Epub 2011 Jul 18. Proc Natl Acad Sci U S A. 2011. PMID: 21768350 Free PMC article.
-
Sequences of two related multiple antibiotic resistance virulence plasmids sharing a unique IS26-related molecular signature isolated from different Escherichia coli pathotypes from different hosts.PLoS One. 2013 Nov 4;8(11):e78862. doi: 10.1371/journal.pone.0078862. eCollection 2013. PLoS One. 2013. PMID: 24223859 Free PMC article.
References
-
- Benjelloun-Touimi, Z., P. J. Sansonetti, and C. Parsot. 1995. SepA, the major extracellular protein of Shigella flexneri: autonomous secretion and involvement in tissue invasion. Mol. Microbiol. 17:123-135. - PubMed
-
- Brunder, W., H. Schmidt, M. Frosch, and H. Karch. 1999. The large plasmids of Shiga-toxin-producing Escherichia coli (STEC) are highly variable genetic elements. Microbiology 145:1005-1014. - PubMed
-
- Brunder, W., H. Schmidt, and H. Karch. 1997. EspP, a novel extracellular serine protease of enterohaemorrhagic Escherichia coli O157:H7 cleaves human coagulation factor V. Mol. Microbiol. 24:767-778. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

