Relative immunogenicity of PorA subtypes in a multivalent Neisseria meningitidis vaccine is not dependent on presentation form
- PMID: 14573657
- PMCID: PMC219571
- DOI: 10.1128/IAI.71.11.6367-6371.2003
Relative immunogenicity of PorA subtypes in a multivalent Neisseria meningitidis vaccine is not dependent on presentation form
Abstract
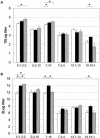
The hexavalent meningococcal vaccine HexaMen, containing six PorAs on two vesicles, was tested in clinical studies. Although fourfold increases in serum bactericidal activity (SBA) titers against all of the PorAs were observed, there were significant differences between PorA-specific SBA titers. SBA titers were mainly directed against one PorA from each vesicle, P1.5-2,10 and P1.5-1,2-2, and were lower against the other PorAs, especially P1.7-2,4 and P1.19,15-1. We investigated whether these differences were due to immunological interference that resulted in competition between the three PorAs on the same vesicle or whether they were caused by a difference in the immunogenicities of the separate PorAs. Therefore, mice were immunized either with HexaMen, with six monovalent outer membrane vesicles (OMVs) representing the same six PorAs simultaneously (HexaMix), or with only one of the monovalent OMVs. The immunoglobulin G and SBA titers after HexaMen immunization in mice resembled the results obtained in clinical studies. Although immunization with HexaMix gave higher titers than immunization with HexaMen for some PorAs, the pattern of high and low titers was the same. Similar differences in immunogenicity between subtypes were seen after monovalent immunization when interference was eliminated as a cause of the differences. Monovalent immunization resulted in higher titers for P1.5-1,2-2 and P1.7,16 than immunization with HexaMen. However, no significant differences were found for the weakly immunogenic PorAs, P1.7-2,4 and P1.19,15-1. Since immunization with the six PorAs in the trivalent presentation form (HexaMen) and in the mixture of monovalent vesicles (HexaMix) resulted in the same pattern of high and low titers, we concluded that the differences between the PorA-specific responses are due to differences in the immunogenicities of the various PorAs and not due to interference that results in competition between different PorAs.
Figures
Similar articles
-
Hyperproliferation of B cells specific for a weakly immunogenic PorA in a meningococcal vaccine model.Clin Vaccine Immunol. 2008 Oct;15(10):1598-605. doi: 10.1128/CVI.00192-08. Epub 2008 Sep 3. Clin Vaccine Immunol. 2008. PMID: 18768670 Free PMC article.
-
Heterologous prime-boost strategy to overcome weak immunogenicity of two serosubtypes in hexavalent Neisseria meningitidis outer membrane vesicle vaccine.Vaccine. 2006 Mar 6;24(10):1569-77. doi: 10.1016/j.vaccine.2005.10.003. Epub 2005 Oct 24. Vaccine. 2006. PMID: 16298029
-
PorA-specific differences in antibody avidity after vaccination with a hexavalent Men B outer membrane vesicle vaccine in toddlers and school children.Vaccine. 2004 Aug 13;22(23-24):3008-13. doi: 10.1016/j.vaccine.2004.02.014. Vaccine. 2004. PMID: 15297049 Clinical Trial.
-
Cross-reactivity of antibodies against PorA after vaccination with a meningococcal B outer membrane vesicle vaccine.Infect Immun. 2003 Apr;71(4):1650-5. doi: 10.1128/IAI.71.4.1650-1655.2003. Infect Immun. 2003. PMID: 12654777 Free PMC article. Clinical Trial.
-
Human B- and T-cell responses after immunization with a hexavalent PorA meningococcal outer membrane vesicle vaccine.Infect Immun. 1997 Dec;65(12):5184-90. doi: 10.1128/iai.65.12.5184-5190.1997. Infect Immun. 1997. PMID: 9393814 Free PMC article.
Cited by
-
Improved production process for native outer membrane vesicle vaccine against Neisseria meningitidis.PLoS One. 2013 May 31;8(5):e65157. doi: 10.1371/journal.pone.0065157. Print 2013. PLoS One. 2013. PMID: 23741478 Free PMC article.
-
Coincorporation of LpxL1 and PagL mutant lipopolysaccharides into liposomes with Neisseria meningitidis opacity protein: influence on endotoxic and adjuvant activity.Clin Vaccine Immunol. 2010 Apr;17(4):487-95. doi: 10.1128/CVI.00423-09. Epub 2010 Jan 27. Clin Vaccine Immunol. 2010. PMID: 20107001 Free PMC article.
-
Differential effect of TLR2 and TLR4 on the immune response after immunization with a vaccine against Neisseria meningitidis or Bordetella pertussis.PLoS One. 2010 Dec 23;5(12):e15692. doi: 10.1371/journal.pone.0015692. PLoS One. 2010. PMID: 21203418 Free PMC article.
-
A novel meningococcal outer membrane vesicle vaccine with constitutive expression of FetA: A phase I clinical trial.J Infect. 2015 Sep;71(3):326-37. doi: 10.1016/j.jinf.2015.05.006. Epub 2015 May 15. J Infect. 2015. PMID: 25982025 Free PMC article. Clinical Trial.
-
Hyperproliferation of B cells specific for a weakly immunogenic PorA in a meningococcal vaccine model.Clin Vaccine Immunol. 2008 Oct;15(10):1598-605. doi: 10.1128/CVI.00192-08. Epub 2008 Sep 3. Clin Vaccine Immunol. 2008. PMID: 18768670 Free PMC article.
References
-
- Boslego, J., J. Garcia, C. Cruz, W. Zollinger, B. Brandt, S. Ruiz, M. Martinez, J. Arthur, P. Underwood, and W. Silva. 1995. Efficacy, safety, and immunogenicity of a meningococcal group B (15:P1.3) outer membrane protein vaccine in Iquique, Chile. Chilean National Committee for Meningococcal Disease. Vaccine 13:821-829. - PubMed
-
- Cartwright, K., R. Morris, H. Rumke, A. Fox, R. Borrow, N. Begg, P. Richmond, and J. Poolman. 1999. Immunogenicity and reactogenicity in UK infants of a novel meningococcal vesicle vaccine containing multiple class 1 (PorA) outer membrane proteins. Vaccine 17:2612-2619. - PubMed
-
- Cartwright, K., N. Noah, and H. Peltola. 2001. Meningococcal disease in Europe: epidemiology, mortality, and prevention with conjugate vaccines. Report of a European advisory board meeting, Vienna, Austria, 6-8 October, 2000. Vaccine 19:4347-4356. - PubMed
-
- Claassen, I., J. Meylis, P. van der Ley, C. Peeters, H. Brons, J. Robert, D. Borsboom, A. van der Ark, I. van Straaten, P. Roholl, B. Kuipers, and J. Poolman. 1996. Production, characterization and control of a Neisseria meningitidis hexavalent class 1 outer membrane protein containing vesicle vaccine. Vaccine 14:1001-1008. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

