Inhibition of the complement membrane attack complex by Schistosoma mansoni paramyosin
- PMID: 14573661
- PMCID: PMC219572
- DOI: 10.1128/IAI.71.11.6402-6410.2003
Inhibition of the complement membrane attack complex by Schistosoma mansoni paramyosin
Abstract
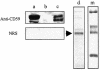
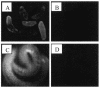
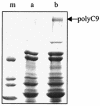
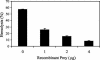
Larvae and adults of the parasitic blood fluke Schistosoma mansoni are resistant to killing by human complement. An earlier search by Parizade et al. for a schistosome complement inhibitor identified a 94-kDa surface protein which was named SCIP-1 (M. Parizade, R. Arnon, P. J. Lachmann, and Z. Fishelson, J. Exp. Med. 179:1625-1636, 1994). Following partial purification and analysis by mass spectrometry, we have determined SCIP-1 to be a surface-exposed form of the muscle protein paramyosin. As shown by immunofluorescence, anti-paramyosin antibodies label the surface of live schistosomula and adult worms. Like SCIP-1, purified native paramyosin reacts with a polyclonal rabbit anti-human CD59 antiserum, as shown by Western blot analysis. Also, the human complement components C8 and C9 bind to recombinant and native paramyosin. Analysis of paramyosin binding to fragments of C9 generated by thrombin or trypsin has demonstrated that paramyosin binds to C9 at a position located between Gly245 and Arg391. Paramyosin inhibited Zn(2+)-induced C9 polymerization and poly-C9 deposition onto rabbit erythrocytes (E(R)). In addition, paramyosin inhibited lysis of E(R) and of sensitized sheep erythrocytes by human complement. Finally, anti-paramyosin antibodies enhanced in vitro killing of schistosomula by normal and C4-depleted human complement. Taken together, these findings suggest that an exogenous form of S. mansoni paramyosin inhibits activation of the terminal pathway of complement and thus has an important immunomodulatory role in schistosomiasis.
Figures






Similar articles
-
Mapping of the complement C9 binding domain in paramyosin of the blood fluke Schistosoma mansoni.Int J Parasitol. 2007 Jan;37(1):67-75. doi: 10.1016/j.ijpara.2006.09.011. Epub 2006 Oct 19. Int J Parasitol. 2007. PMID: 17123534
-
Trichinella spiralis paramyosin binds to C8 and C9 and protects the tissue-dwelling nematode from being attacked by host complement.PLoS Negl Trop Dis. 2011 Jul;5(7):e1225. doi: 10.1371/journal.pntd.0001225. Epub 2011 Jul 5. PLoS Negl Trop Dis. 2011. PMID: 21750743 Free PMC article.
-
Functional and antigenic similarities between a 94-kD protein of Schistosoma mansoni (SCIP-1) and human CD59.J Exp Med. 1994 May 1;179(5):1625-36. doi: 10.1084/jem.179.5.1625. J Exp Med. 1994. PMID: 7513011 Free PMC article.
-
Mechanisms of evasion of Schistosoma mansoni schistosomula to the lethal activity of complement.Mem Inst Oswaldo Cruz. 1992;87 Suppl 4:111-6. doi: 10.1590/s0074-02761992000800016. Mem Inst Oswaldo Cruz. 1992. PMID: 1285336 Review.
-
Update on paramyosin in parasitic worms.Parasitol Int. 2005 Jun;54(2):101-7. doi: 10.1016/j.parint.2005.02.004. Epub 2005 Apr 11. Parasitol Int. 2005. PMID: 15866471 Review.
Cited by
-
Sm16, A Schistosoma mansoni Immunomodulatory Protein, Fails to Elicit a Protective Immune Response and Does Not Have an Essential Role in Parasite Survival in the Definitive Host.J Immunol Res. 2019 Dec 1;2019:6793596. doi: 10.1155/2019/6793596. eCollection 2019. J Immunol Res. 2019. PMID: 31886307 Free PMC article.
-
Among-population proteomic differences in Schistocephalus solidus based on excretory/secretory and total body protein predictions.Parasit Vectors. 2025 May 20;18(1):180. doi: 10.1186/s13071-025-06807-x. Parasit Vectors. 2025. PMID: 40394694 Free PMC article.
-
Mapping of the Complement C9 Binding Region on Clonorchis sinensis Paramyosin.Korean J Parasitol. 2022 Aug;60(4):255-259. doi: 10.3347/kjp.2022.60.4.255. Epub 2022 Aug 24. Korean J Parasitol. 2022. PMID: 36041487 Free PMC article.
-
Screening and molecular cloning of a protective antigen from the midgut of Haemaphysalis longicornis.Korean J Parasitol. 2013 Jun;51(3):327-34. doi: 10.3347/kjp.2013.51.3.327. Epub 2013 Jun 30. Korean J Parasitol. 2013. PMID: 23864744 Free PMC article.
-
Mapping of the complement C9 binding domain on Trichinella spiralis paramyosin.Parasit Vectors. 2014 Feb 24;7:80. doi: 10.1186/1756-3305-7-80. Parasit Vectors. 2014. PMID: 24564979 Free PMC article.
References
-
- Becker, M. M., B. H. Kalinna, W. Yang, S. A. Harrop, J. C. Scott, G. J. Waine, J. D. Kurtis, and D. P. McManus. 1995. Gene cloning and complete nucleotide sequence of Philippine Schistosoma japonicum paramyosin. Acta Trop. 59:143-147. - PubMed
-
- Bergquist, R., M. Al-Sherbiny, R. Barakat, and R. Olds. 2002. Blueprint for schistosomiasis vaccine development. Acta Trop. 82:183-192. - PubMed
-
- Biesecker, G., C. Gerard, and T. E. Hugli. 1982. An amphiphilic structure of the ninth component of human complement. Evidence from analysis of fragments produced by alpha-thrombin. J. Biol. Chem. 257:2584-2590. - PubMed
-
- Brink, L. H., D. J. McLaren, and S. R. Smithers. 1977. Schistosoma mansoni: a comparative study of artificially transformed schistosomula and schistosomula recovered after cercarial penetration of isolated skin. Parasitology 74:73-86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

