Recombinant Leishmania major secreting biologically active granulocyte-macrophage colony-stimulating factor survives poorly in macrophages in vitro and delays disease development in mice
- PMID: 14573672
- PMCID: PMC219543
- DOI: 10.1128/IAI.71.11.6499-6509.2003
Recombinant Leishmania major secreting biologically active granulocyte-macrophage colony-stimulating factor survives poorly in macrophages in vitro and delays disease development in mice
Abstract
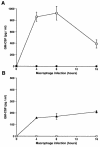
Leishmania is an intracellular pathogen that replicates inside macrophages. Activated macrophages produce a specific subset of cytokines that play an important role in the control of Leishmania infections. As part of our interest in developing suicide parasites that produce abortive infections for the purposes of vaccination, we engineered recombinant Leishmania major strains producing biologically active granulocyte-macrophage colony-stimulating factor (GM-CSF). We showed that GM-CSF is being produced in the phagosomes of infected macrophages and that it can be detected in the culture supernatants of both infected macrophages and extracellular parasites. Our data support the notion that GM-CSF secreted by both developmental forms of recombinant L. major can activate macrophages to produce high levels of proinflammatory cytokines such as interleukin-1beta (IL-1beta), IL-6, and IL-18 and various chemokines including RANTES/CCL5, MIP-1alpha/CCL3, MIP-1beta/CCL4, MIP-2/CXCL2, and MCP-1/CCL2, which enhance parasite killing. Indeed, GM-CSF-expressing parasites survive poorly in macrophages in vitro and produce delayed lesion development in susceptible BALB/c mice in vivo. Selective killing of intracellular Leishmania expressing cytokine genes capable of activating cellular responses may constitute a promising strategy to control and/or prevent parasitic infections.
Figures




Similar articles
-
Susceptibility or resistance to Leishmania infection is dictated by the macrophages evolved under the influence of IL-3 or GM-CSF.Eur J Immunol. 1999 Jul;29(7):2319-29. doi: 10.1002/(SICI)1521-4141(199907)29:07<2319::AID-IMMU2319>3.0.CO;2-3. Eur J Immunol. 1999. PMID: 10427995
-
Involvement of mannose receptor in cytokine interleukin-1beta (IL-1beta), IL-6, and granulocyte-macrophage colony-stimulating factor responses, but not in chemokine macrophage inflammatory protein 1beta (MIP-1beta), MIP-2, and KC responses, caused by attachment of Candida albicans to macrophages.Infect Immun. 1997 Mar;65(3):1077-82. doi: 10.1128/IAI.65.3.1077-1082.1997. Infect Immun. 1997. PMID: 9038318 Free PMC article.
-
Mice unresponsive to GM-CSF are unexpectedly resistant to cutaneous Leishmania major infection.Microbes Infect. 2000 Aug;2(10):1131-8. doi: 10.1016/s1286-4579(00)01267-3. Microbes Infect. 2000. PMID: 11008103
-
The effect of granulocyte-macrophage colony stimulating factor (rGM-CSF) on macrophage function in microbial disease.Med Oncol. 1996 Sep;13(3):141-7. doi: 10.1007/BF02990842. Med Oncol. 1996. PMID: 9106172 Free PMC article. Review.
-
Metabolic stringent response in intracellular stages of Leishmania.Curr Opin Microbiol. 2021 Oct;63:126-132. doi: 10.1016/j.mib.2021.07.007. Epub 2021 Jul 31. Curr Opin Microbiol. 2021. PMID: 34340099 Review.
Cited by
-
CXCL10/gamma interferon-inducible protein 10-mediated protection against Leishmania amazonensis infection in mice.Infect Immun. 2006 Dec;74(12):6769-77. doi: 10.1128/IAI.01073-06. Epub 2006 Sep 18. Infect Immun. 2006. PMID: 16982826 Free PMC article.
-
The transition of M-CSF-derived human macrophages to a growth-promoting phenotype.Blood Adv. 2020 Nov 10;4(21):5460-5472. doi: 10.1182/bloodadvances.2020002683. Blood Adv. 2020. PMID: 33166408 Free PMC article.
-
Transgenic Leishmania and the immune response to infection.Parasite Immunol. 2008 Apr;30(4):255-66. doi: 10.1111/j.1365-3024.2008.01020.x. Epub 2008 Feb 4. Parasite Immunol. 2008. PMID: 18266814 Free PMC article. Review.
-
Development of Leishmania vaccines: predicting the future from past and present experience.J Biomed Res. 2013 Mar;27(2):85-102. doi: 10.7555/JBR.27.20120064. Epub 2012 Sep 30. J Biomed Res. 2013. PMID: 23554800 Free PMC article.
-
An 8-hydroxyquinoline-containing polymeric micelle system is effective for the treatment of murine tegumentary leishmaniasis.Parasitol Res. 2016 Nov;115(11):4083-4095. doi: 10.1007/s00436-016-5181-4. Epub 2016 Jul 1. Parasitol Res. 2016. PMID: 27365053
References
-
- Alvaro-Gracia, J. M., N. J. Zvaifler, and G. S. Firestein. 1989. Cytokines in chronic inflammatory arthritis. IV. Granulocyte/macrophage colony-stimulating factor-mediated induction of class II MHC antigen on human monocytes: a possible role in rheumatoid arthritis. J. Exp. Med. 170:865-875. - PMC - PubMed
-
- Al-Zamel, F., F. J. Al-Shammary, S. El-Shewemi, and R. Soliman. 1996. Enhancement of leishmanicidal activity of human macrophages against Leishmania major and Leishmania donovani infection using recombinant human granulocyte macrophage colony stimulating factor. Zentbl. Bakteriol. 285:92-105. - PubMed
-
- Arend, W. P., M. Malyak, C. J. Guthridge, and C. Gabay. 1998. Interleukin-1 receptor antagonist: role in biology. Annu. Rev. Immunol. 16:27-55. - PubMed
-
- Armitage, J. O. 1998. Emerging applications of recombinant human granulocyte-macrophage colony-stimulating factor. Blood 92:4491-4508. - PubMed
-
- Badaro, R., C. Nascimento, J. S. Carvalho, F. Badaro, D. Russo, J. L. Ho, S. G. Reed, W. D. Johnson, Jr., and T. C. Jones. 1994. Granulocyte-macrophage colony-stimulating factor in combination with pentavalent antimony for the treatment of visceral leishmaniasis. Eur. J. Clin. Microbiol. Infect. Dis. 13 (Suppl. 2):S23-S28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

