Forssman synthetase expression results in diminished shiga toxin susceptibility: a role for glycolipids in determining host-microbe interactions
- PMID: 14573676
- PMCID: PMC219581
- DOI: 10.1128/IAI.71.11.6543-6552.2003
Forssman synthetase expression results in diminished shiga toxin susceptibility: a role for glycolipids in determining host-microbe interactions
Abstract
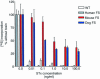
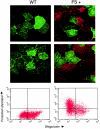
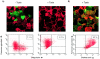
Forssman glycolipid (FG), the product of Forssman synthetase (FS), is widely expressed among nonprimate mammalian species. Here, we describe a molecular and genetic relationship between FG expression and Shiga toxin (Stx) susceptibility. We have isolated the FS cDNA from human, canine, and murine cells. Whereas the murine and canine FS genes express a functional enzyme, the human FS cDNA was found to express a protein that lacks FS activity, despite a high degree of sequence identity with the enzymatically active murine and canine FS genes. In order to examine the relationship between FG expression and Stx susceptibility, Vero cells were transfected with the three FS orthologues or a vector control. Complementation with the human FS cDNA had no effect on Stx susceptibility, whereas stable expression of the canine and murine FS resulted in markedly decreased susceptibility to toxin. Among individual cells, an inverse correlation between FG expression and Stx binding was demonstrated. Moreover, only strongly FG-reactive cells were capable of growing in the presence of Stx. These cells were found to have high levels of FG expression and a correspondingly diminished GbO(3) content. We conclude that expression of a functionally active FS modifies Stx receptor glycolipids to FG and results in markedly decreased susceptibility to toxin. We speculate that inactivation of the FS gene during primate evolution may account, at least in part, for the marked susceptibility of human cells to Stx.
Figures


References
-
- Andrews, P. W., E. Nudelman, S. Hakomori, and B. A. Fenderson. 1990. Different patterns of glycolipid antigens are expressed following differentiation of TERA-2 human embryonal carcinoma cells induced by retinoic acid, hexamethylene bisacetamide (HMBA) or bromodeoxyuridine (BUdR). Differentiation 43:131-138. - PubMed
-
- Beachey, E. H. 1981. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surfaces. J. Infect. Dis. 143:325-345. - PubMed
-
- Besser, T. E., D. D. Hancock, L. C. Pritchett, E. M. McRae, D. H. Rice, and P. I. Tarr. 1997. Duration of detection of fecal excretion of Escherichia coli O157:H7 in cattle. J. Infect. Dis. 175:726-729. - PubMed
-
- Beutin, L., D. Geier, S. Zimmermann, S. Aleksic, H. A. Gillespie, and T. S. Whittam. 1997. Epidemiological relatedness and clonal types of natural populations of Escherichia coli strains producing Shiga toxins in separate populations of cattle and sheep. Appl. Environ. Microbiol. 63:2175-2180. - PMC - PubMed
-
- Cartron, J. P., and Y. Colin. 2001. Structural and functional diversity of blood group antigens. Transfus. Clin. Biol. 8:163-199. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

