Inhibition of platelet adherence to brain microvasculature protects against severe Plasmodium berghei malaria
- PMID: 14573677
- PMCID: PMC219602
- DOI: 10.1128/IAI.71.11.6553-6561.2003
Inhibition of platelet adherence to brain microvasculature protects against severe Plasmodium berghei malaria
Abstract
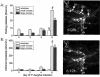
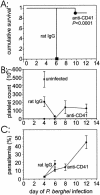
Some patients with Plasmodium falciparum infections develop cerebral malaria, acute respiratory distress, and shock and ultimately die even though drug therapy has eliminated the parasite from the blood, suggesting that a systemic inflammatory response contributes to malarial pathogenesis. Plasmodium berghei-infected mice are a well-recognized model of severe malaria (experimental severe malaria [ESM]), and infected mice exhibit a systemic inflammatory response. Because platelets are proposed to contribute to ESM and other systemic inflammatory responses, we determined whether platelet adherence contributes to experimental malarial pathogenesis. Indeed, a significant (P < 0.005) increase in the number of rolling and adherent platelets was observed by intravital microscopy in brain venules of P. berghei-infected mice compared with the number in uninfected controls. P-selectin- or ICAM-1-deficient mice exhibit increased survival after P. berghei infection. We observed a significant (P < 0.0001) reduction in the morbidity of mice injected with anti-CD41 (alpha(IIb) or gpIIb) monoclonal antibody on day 1 of P. berghei infection compared with the morbidity of infected controls injected with rat immunoglobulin G. Additionally, platelet rolling and adhesion in brain venules were reduced in P. berghei mice lacking either P-selectin or ICAM-1 or when the platelets were coated with anti-CD41 monoclonal antibody. Unlike other inflammatory conditions, we did not detect platelet-leukocyte interactions during P. berghei malaria. Because (i). leukocyte adhesion is not markedly altered in the absence of P-selectin or ICAM-1 and (ii). CD41 is not an adhesion molecule for parasitized erythrocytes, these findings support the hypothesis that inhibition of platelet adhesion to the brain microvasculature protects against development of malarial pathogenesis.
Figures



Similar articles
-
P-selectin contributes to severe experimental malaria but is not required for leukocyte adhesion to brain microvasculature.Infect Immun. 2003 Apr;71(4):1911-8. doi: 10.1128/IAI.71.4.1911-1918.2003. Infect Immun. 2003. PMID: 12654808 Free PMC article.
-
Intercellular adhesion molecule 1 is important for the development of severe experimental malaria but is not required for leukocyte adhesion in the brain.J Investig Med. 2003 May;51(3):128-40. doi: 10.1136/jim-51-03-15. J Investig Med. 2003. PMID: 12769195
-
Platelet depletion by anti-CD41 (alphaIIb) mAb injection early but not late in the course of disease protects against Plasmodium berghei pathogenesis by altering the levels of pathogenic cytokines.Blood. 2005 Mar 1;105(5):1956-63. doi: 10.1182/blood-2004-06-2206. Epub 2004 Oct 19. Blood. 2005. PMID: 15494426
-
Exogenous nitric oxide decreases brain vascular inflammation, leakage and venular resistance during Plasmodium berghei ANKA infection in mice.J Neuroinflammation. 2011 Jun 7;8:66. doi: 10.1186/1742-2094-8-66. J Neuroinflammation. 2011. PMID: 21649904 Free PMC article.
-
Regulation of endothelial cell adhesion molecule expression in an experimental model of cerebral malaria.Microcirculation. 2002 Dec;9(6):463-70. doi: 10.1038/sj.mn.7800159. Microcirculation. 2002. PMID: 12483543
Cited by
-
Platelet activation by Histophilus somni and its lipooligosaccharide induces endothelial cell proinflammatory responses and platelet internalization.Shock. 2008 Feb;29(2):189-96. Shock. 2008. PMID: 18386389 Free PMC article.
-
Sequestration and tissue accumulation of human malaria parasites: can we learn anything from rodent models of malaria?PLoS Pathog. 2010 Sep 30;6(9):e1001032. doi: 10.1371/journal.ppat.1001032. PLoS Pathog. 2010. PMID: 20941396 Free PMC article. Review.
-
The Impact of HIV Coinfection on Cerebral Malaria Pathogenesis.J Neuroparasitology. 2012;3:235547. doi: 10.4303/jnp/235547. Epub 2012 Mar 2. J Neuroparasitology. 2012. PMID: 22545215 Free PMC article.
-
Platelets potentiate brain endothelial alterations induced by Plasmodium falciparum.Infect Immun. 2006 Jan;74(1):645-53. doi: 10.1128/IAI.74.1.645-653.2006. Infect Immun. 2006. PMID: 16369021 Free PMC article.
-
Inflammatory responses associated with the induction of cerebral malaria: lessons from experimental murine models.PLoS Pathog. 2012 Dec;8(12):e1003045. doi: 10.1371/journal.ppat.1003045. Epub 2012 Dec 27. PLoS Pathog. 2012. PMID: 23300435 Free PMC article. No abstract available.
References
-
- Bienvenu, K., and D. N. Granger. 1993. Molecular determinants of shear rate-dependent leukocyte adhesion in postcapillary venules. Am. J. Physiol. 264:H1504-H1508. - PubMed
-
- Chang, W. L., H. C. van der Heyde, and B. S. Klein. 1998. Flow cytometric quantitation of yeast: a novel technique for use in animal model work and in vitro immunologic assays. J. Immunol. Methods 211:51-63. - PubMed
-
- Curfs, J. H., C. C. Hermsen, P. Kremsner, S. Neifer, J. H. Meuwissen, N. Van Rooyen, and W. M. Eling. 1993. Tumour necrosis factor-alpha and macrophages in Plasmodium berghei-induced cerebral malaria. Parasitology 107:125-134. - PubMed
-
- de Kossodo, S., and G. E. Grau. 1993. Profiles of cytokine production in relation with susceptibility to cerebral malaria. J. Immunol. 151:4811-4820. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

