In vitro biological activities of transmembrane superantigen staphylococcal enterotoxin A fusion protein
- PMID: 14574492
- PMCID: PMC11032846
- DOI: 10.1007/s00262-003-0437-0
In vitro biological activities of transmembrane superantigen staphylococcal enterotoxin A fusion protein
Abstract
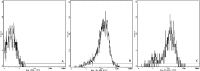
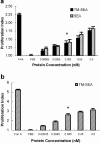
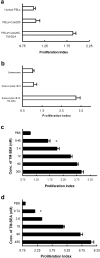
The bacterial superantigen staphylococcal enterotoxin A (SEA) stimulates T cells bearing certain TCR Vbeta domains when binding to MHC II molecules, and is a potent inducer of CTL activity and cytokine production. Antibody-targeted SEA such as C215 Fab-SEA and C242 Fab-SEA has been investigated for cancer therapy in recent years. We have previously reported significant tumor inhibition and prolonged survival time in tumor-bearing mice treated with a combination of both C215Fab-SEA and Ad IL-18 (Wang et al., Gene Therapy 8:542-550, 2001). In order to develop SEA as an universal biological preparation in cancer therapy, we first cloned a SEA gene from S. aureus (ATCC 13565) and a transmembrane (TM) sequence from a c- erb-b2 gene derived from human ovarian cancer cell line HO-8910, then generated a TM-SEA fusion gene by using the splice overlap extension method, and constructed the recombinant expression vector pET-28a-TM-SEA. Fusion protein TM-SEA was expressed in E. coli BL21(DE3)pLysS and purified by using the histidine tag in this vector. Purified TM-SEA spontaneously associated with cell membranes as detected by flow cytometry. TM-SEA stimulated the proliferation of both human PBLs and splenocytes derived from C57BL/6 (H-2b) mice in vitro. This study thus demonstrated a novel strategy for anchoring superantigen SEA onto the surfaces of tumor cells without any genetic manipulation.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

