Quinine pharmacokinetic-pharmacodynamic relationships in uncomplicated falciparum malaria
- PMID: 14576102
- PMCID: PMC253804
- DOI: 10.1128/AAC.47.11.3458-3463.2003
Quinine pharmacokinetic-pharmacodynamic relationships in uncomplicated falciparum malaria
Abstract
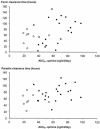
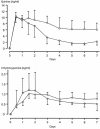
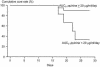
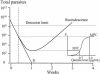
The relationships between the pharmacokinetic properties of quinine during a 7-day treatment course and the therapeutic response were studied in 30 adult patients with uncomplicated falciparum malaria monitored for > or = 28 days. All patients received a 7-day oral quinine regimen either alone (n = 22) or in combination with rifampin (n = 8). The median fever clearance time was 58.5 h, and the mean +/- standard deviation parasite clearance time was 73 +/- 24 h. After recovery, six patients had recrudescences of Plasmodium falciparum malaria and seven had delayed appearances of P. vivax infection between days 16 and 23. Between the patients with and without recrudescences, there were no significant differences either in fever clearance time or parasite clearance time or in the overall pharmacokinetics of quinine and 3-hydroxyquinine. Patients for whom the area under the concentration-time curve from 3 to 7 days for quinine in plasma was <20 microg.day/ml had a relative risk of 5.3 (95% confidence interval = 1.6 to 17.7) of having a subsequent recrudescence of infection (P = 0.016). Modeling of these data suggested an average minimum parasiticidal concentration of quinine in plasma of 3.4 microg/ml and an MIC of 0.7 microg/ml for uncomplicated falciparum malaria in Thailand. To ensure a cure, the minimum parasiticidal concentration must be exceeded during four asexual cycles (>6 days).
Figures

Similar articles
-
Adverse effect of rifampin on quinine efficacy in uncomplicated falciparum malaria.Antimicrob Agents Chemother. 2003 May;47(5):1509-13. doi: 10.1128/AAC.47.5.1509-1513.2003. Antimicrob Agents Chemother. 2003. PMID: 12709315 Free PMC article. Clinical Trial.
-
Effects of cigarette smoking on quinine pharmacokinetics in malaria.Eur J Clin Pharmacol. 2002 Aug;58(5):315-9. doi: 10.1007/s00228-002-0447-4. Epub 2002 Jun 20. Eur J Clin Pharmacol. 2002. PMID: 12185554 Clinical Trial.
-
Comparison of oral artesunate and quinine plus tetracycline in acute uncomplicated falciparum malaria.Bull World Health Organ. 1994;72(2):233-8. Bull World Health Organ. 1994. PMID: 8205643 Free PMC article. Clinical Trial.
-
[Optimum quinine therapy in falciparum malaria attacks contracted in Africa].Bull Soc Pathol Exot. 1997;90(4):260-2. Bull Soc Pathol Exot. 1997. PMID: 9479465 Review. French.
-
Clinical pharmacokinetics of quinine and its relationship with treatment outcomes in children, pregnant women, and elderly patients, with uncomplicated and complicated malaria: a systematic review.Malar J. 2022 Feb 10;21(1):41. doi: 10.1186/s12936-022-04065-1. Malar J. 2022. PMID: 35144612 Free PMC article.
Cited by
-
Interaction between rifampicin, amodiaquine and artemether in mice infected with chloroquine resistant Plasmodium berghei.Malar J. 2014 Aug 5;13:299. doi: 10.1186/1475-2875-13-299. Malar J. 2014. PMID: 25091936 Free PMC article.
-
Population pharmacokinetic and pharmacodynamic properties of intramuscular quinine in Tanzanian children with severe Falciparum malaria.Antimicrob Agents Chemother. 2013 Feb;57(2):775-83. doi: 10.1128/AAC.01349-12. Epub 2012 Nov 26. Antimicrob Agents Chemother. 2013. PMID: 23183442 Free PMC article. Clinical Trial.
-
World Antimalarial Resistance Network (WARN) IV: clinical pharmacology.Malar J. 2007 Sep 6;6:122. doi: 10.1186/1475-2875-6-122. Malar J. 2007. PMID: 17822537 Free PMC article.
-
Pharmacokinetic and pharmacodynamic considerations in antimalarial dose optimization.Antimicrob Agents Chemother. 2013 Dec;57(12):5792-807. doi: 10.1128/AAC.00287-13. Epub 2013 Sep 3. Antimicrob Agents Chemother. 2013. PMID: 24002099 Free PMC article. Review.
-
In vivo assessment of drug efficacy against Plasmodium falciparum malaria: duration of follow-up.Antimicrob Agents Chemother. 2004 Nov;48(11):4271-80. doi: 10.1128/AAC.48.11.4271-4280.2004. Antimicrob Agents Chemother. 2004. PMID: 15504852 Free PMC article.
References
-
- Brockman, A., R. N. Price, M. van Vugt, D. G. Heppner, D. Walsh, P. Sookto, T. Wimonwattrawatee, S. Looareesuwan, N. J. White, and F. Nosten. 2000. Plasmodium falciparum antimalarial drug susceptibility on the northwestern border of Thailand during five years of extensive artesunate-mefloquine use. Trans. R. Soc. Trop. Med. Hyg. 94:537-544. - PMC - PubMed
-
- Chongsuphajaisiddhi, T., A. Sabcharoen, and P. Attanath. 1981. In vivo and in vitro sensitivity of falciparum malaria to quinine in Thai children. Ann. Trop. Paediatr. i:21-26. - PubMed
-
- Earle, D. P., R. W. Berliner, J. V. Taggart, W. J. Welch, C. G. Zubrod, N. Bowman-Wise, T. C. Chalmers, R. L. Grief, and J. A. Shannon. 1948. Studies on the chemotherapy of the human malarias. II. Method for the quantitative assay of suppressive antimalarial action in falciparum malaria. J. Clin. Investig. 27:75-79. - PMC - PubMed
-
- Looareesuwan, S., P. Wilairatana, S. Vanijanonta, D. Kyle, and K. Webster. 1992. Efficacy of quinine-tetracycline for acute uncomplicated falciparum malaria in Thailand. Lancet i:367-370. - PubMed
-
- Nontprasert, A., S. Pukrittayakamee, D. E. Kyle, S. Vanijanonta, and N. J. White. 1996. Antimalarial activity and interactions between quinine, dihydroquinine, and 3-hydroxyquinine against P. falciparum in vitro. Trans. R. Soc. Trop. Med. Hyg. 90:553-555. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

