Hydralazine for treatment of severe hypertension in pregnancy: meta-analysis
- PMID: 14576246
- PMCID: PMC259162
- DOI: 10.1136/bmj.327.7421.955
Hydralazine for treatment of severe hypertension in pregnancy: meta-analysis
Abstract
Objective: To review outcomes in randomised controlled trials comparing hydralazine against other antihypertensives for severe hypertension in pregnancy.
Study design: Meta-analysis of randomised controlled trials (published between 1966 and September 2002) of short acting antihypertensives for severe hypertension in pregnancy. Independent data abstraction by two reviewers. Data were entered into RevMan software for analysis (fixed effects model, relative risk and 95% confidence interval); in a secondary analysis, risk difference was also calculated.
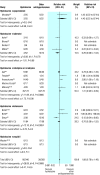
Results: Of 21 trials (893 women), eight compared hydralazine with nifedipine and five with labetalol. Hydralazine was associated with a trend towards less persistent severe hypertension than labetalol (relative risk 0.29 (95% confidence interval 0.08 to 1.04); two trials), but more severe hypertension than nifedipine or isradipine (1.41 (0.95 to 2.09); four trials); there was significant heterogeneity in outcome between trials and differences in methodological quality. Hydralazine was associated with more maternal hypotension (3.29 (1.50 to 7.23); 13 trials); more caesarean sections (1.30 (1.08 to 1.59); 14 trials); more placental abruption (4.17 (1.19 to 14.28); five trials); more maternal oliguria (4.00 (1.22 to 12.50); three trials); more adverse effects on fetal heart rate (2.04 (1.32 to 3.16); 12 trials); and more low Apgar scores at one minute (2.70 (1.27 to 5.88); three trials). For all but Apgar scores, analysis by risk difference showed heterogeneity between trials. Hydralazine was associated with more maternal side effects (1.50 (1.16 to 1.94); 12 trials) and with less neonatal bradycardia than labetalol (risk difference -0.24 (-0.42 to -0.06); three trials).
Conclusions: The results are not robust enough to guide clinical practice, but they do not support use of hydralazine as first line for treatment of severe hypertension in pregnancy. Adequately powered clinical trials are needed, with a comparison of labetalol and nifedipine showing the most promise.
Figures




Similar articles
-
Factor analysis, including antihypertensive medication, of the outcome of pregnancy in pregnancy-associated hypertension.Kidney Blood Press Res. 2001;24(2):124-8. doi: 10.1159/000054218. Kidney Blood Press Res. 2001. PMID: 11435745
-
Different dosage regimens of nifedipine, labetalol, and hydralazine for the treatment of severe hypertension during pregnancy: a network meta-analysis of randomized controlled trials.Hypertens Pregnancy. 2022 May;41(2):126-138. doi: 10.1080/10641955.2022.2056196. Epub 2022 Mar 31. Hypertens Pregnancy. 2022. PMID: 35361052
-
[Clinical efficacy and perinatal outcome of nifedipine for severe preeclampsia: meta-analysis].Zhonghua Fu Chan Ke Za Zhi. 2012 Aug;47(8):592-7. Zhonghua Fu Chan Ke Za Zhi. 2012. PMID: 23141179 Chinese.
-
The flipside of hydralazine in pregnancy: A systematic review and meta-analysis.Pregnancy Hypertens. 2020 Jan;19:177-186. doi: 10.1016/j.preghy.2020.01.011. Epub 2020 Jan 21. Pregnancy Hypertens. 2020. PMID: 32044579
-
Antihypertensive drug therapy for mild to moderate hypertension during pregnancy.Cochrane Database Syst Rev. 2018 Oct 1;10(10):CD002252. doi: 10.1002/14651858.CD002252.pub4. Cochrane Database Syst Rev. 2018. PMID: 30277556 Free PMC article.
Cited by
-
Changes in trends and outcomes of eclampsia: a success story from Qatar.Qatar Med J. 2019 Sep 20;2019(1):10. doi: 10.5339/qmj.2019.10. eCollection 2019. Qatar Med J. 2019. PMID: 34113550 Free PMC article.
-
Nicardipine for treating severe antepartum hypertension during pregnancy: Nine years of experience in more than 800 women.Acta Obstet Gynecol Scand. 2022 Sep;101(9):1017-1025. doi: 10.1111/aogs.14406. Epub 2022 Jun 16. Acta Obstet Gynecol Scand. 2022. PMID: 35707886 Free PMC article.
-
Hypertension in pregnancy.Curr Atheroscler Rep. 2014 Mar;16(3):395. doi: 10.1007/s11883-013-0395-8. Curr Atheroscler Rep. 2014. PMID: 24477794 Review.
-
Retrospective analysis of risk factors of hypotensive bradycardic events during shoulder arthroscopic surgery under interscalene blockade in the sitting position.Korean J Anesthesiol. 2020 Nov;73(6):542-549. doi: 10.4097/kja.20035. Epub 2020 Mar 27. Korean J Anesthesiol. 2020. PMID: 32213804 Free PMC article.
-
The Pathophysiology, Prognosis and Treatment of Hypertension in Females from Pregnancy to Post-menopause: A Review.Curr Heart Fail Rep. 2024 Aug;21(4):322-336. doi: 10.1007/s11897-024-00672-y. Epub 2024 Jun 11. Curr Heart Fail Rep. 2024. PMID: 38861130 Free PMC article. Review.
References
-
- Department of Health. Report on confidential enquiries into maternal deaths in England and Wales 1982-1984. London: HMSO, 1989. - PubMed
-
- Department of Health. Report on confidential enquiries into maternal deaths in the United Kingdom 1985-1987. London: HMSO, 1991.
-
- Department of Health. Report on confidential enquiries into maternal deaths in the United Kingdom 1988-1990. London: HMSO, 1994.
-
- Department of Health. Report on confidential enquiries into maternal deaths in the United Kingdom 1991-1993. London: HMSO, 1996.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
