The role of glutathione-S-transferase in anti-cancer drug resistance
- PMID: 14576844
- PMCID: PMC6361125
- DOI: 10.1038/sj.onc.1206940
The role of glutathione-S-transferase in anti-cancer drug resistance
Abstract
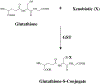
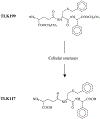
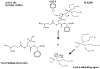
Glutathione-S-transferases (GSTs) are a family of Phase II detoxification enzymes that catalyse the conjugation of glutathione (GSH) to a wide variety of endogenous and exogenous electrophilic compounds. GSTs are divided into two distinct super-family members: the membrane-bound microsomal and cytosolic family members. Microsomal GSTs are structurally distinct from the cytosolic in that they homo- and heterotrimerize rather than dimerize to form a single active site. Microsomal GSTs play a key role in the endogenous metabolism of leukotrienes and prostaglandins. Human cytosolic GSTs are highly polymorphic and can be divided into six classes: alpha, mu, omega, pi, theta, and zeta. The pi and mu classes of GSTs play a regulatory role in the mitogen-activated protein (MAP) kinase pathway that participates in cellular survival and death signals via protein : protein interactions with c-Jun N-terminal kinase 1 (JNK1) and ASK1 (apoptosis signal-regulating kinase). JNK and ASK1 are activated in response to cellular stress. GSTs have been implicated in the development of resistance toward chemotherapy agents. It is plausible that GSTs serve two distinct roles in the development of drug resistance via direct detoxification as well as acting as an inhibitor of the MAP kinase pathway. The link between GSTs and the MAP kinase pathway provides a rationale as to why in many cases the drugs used to select for resistance are neither subject to conjugation with GSH, nor substrates for GSTs. GSTs have emerged as a promising therapeutic target because specific isozymes are overexpressed in a wide variety of tumors and may play a role in the etiology of other diseases, including neurodegenerative diseases, multiple sclerosis, and asthma. Some of the therapeutic strategies so far employed are described in this review.
Figures
References
-
- Bakker J, Lin X and Nelson WG. (2002). J. Biol. Chem, 277, 22573–22580. - PubMed
-
- Brown GL, Lum RT, Schow SR, Maack C, Vollmer C, Gomez R, Delioukina M, Boulos L, Laxa B, Brown J and Rosen LS. (2001). TLK286: phase I dose escalation trial in patients with advanced malignancies.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous