Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors
- PMID: 14578181
- PMCID: PMC1892429
- DOI: 10.1016/S0002-9440(10)63540-7
Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors
Abstract
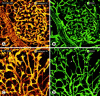
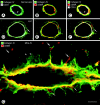
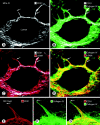
Often described as incomplete or absent, the basement membrane of blood vessels in tumors has attracted renewed attention as a source of angiogenic and anti-angiogenic molecules, site of growth factor binding, participant in angiogenesis, and potential target in cancer therapy. This study evaluated the composition, extent, and structural integrity of the basement membrane on blood vessels in three mouse tumor models: spontaneous RIP-Tag2 pancreatic islet tumors, MCa-IV mammary carcinomas, and Lewis lung carcinomas. Tumor vessels were identified by immunohistochemical staining for the endothelial cell markers CD31, endoglin (CD105), vascular endothelial growth factor receptor-2, and integrin alpha5 (CD49e). Confocal microscopic studies revealed that basement membrane identified by type IV collagen immunoreactivity covered >99.9% of the surface of blood vessels in the three tumors, just as in normal pancreatic islets. Laminin, entactin/nidogen, and fibronectin immunoreactivities were similarly ubiquitous on tumor vessels. Holes in the basement membrane, found by analyzing 1- micro m confocal optical sections, were <2.5 micro m in diameter and involved only 0.03% of the vessel surface. Despite the extensive vessel coverage, the basement membrane had conspicuous structural abnormalities, including a loose association with endothelial cells and pericytes, broad extensions away from the vessel wall, and multiple layers visible by electron microscopy. Type IV collagen-immunoreactive sleeves were also present on endothelial sprouts, supporting the idea that basement membrane is present where sprouts grow and regress. These findings indicate that basement membrane covers most tumor vessels but has profound structural abnormalities, consistent with the dynamic nature of endothelial cells and pericytes in tumors.
Figures




References
-
- Pasqualini R, Arap W, McDonald DM: Probing the structural and molecular diversity of tumor vasculature. Trends Mol Med 2002, 8:563-571 - PubMed
-
- St. Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW: Genes expressed in human tumor endothelium. Science 2000, 289:1197-1202 - PubMed
-
- Folkman J: Angiogenesis-dependent diseases. Semin Oncol 2001, 28:536-542 - PubMed
-
- Jain RK: Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med 2001, 7:987-989 - PubMed
-
- Warren BA: The vascular morphology of tumors. Peterson HE eds. Tumor Blood Circulation. 1979:pp 1-48 CRC Press Inc., Boca Raton
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

