In vivo interrogation of the molecular display of atherosclerotic lesion surfaces
- PMID: 14578186
- PMCID: PMC1892421
- DOI: 10.1016/S0002-9440(10)63545-6
In vivo interrogation of the molecular display of atherosclerotic lesion surfaces
Abstract
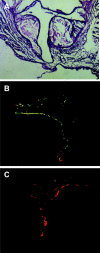
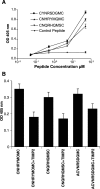
The endothelial surface of atherosclerotic lesions of ApoE knockout mice was interrogated by in vivo biopanning with a phage-displayed constrained peptidyl library. Through repeated biopanning, 103 peptidyl sequences were identified, many are homologous to known proteins. The sequence CAPGPSKSC contains motifs that are shared by 9.7% of selected peptides. On phage or as a synthetic peptide, this constrained peptide selectively bound to atherosclerotic lesion surfaces of ApoE knockout mice in vivo and of human atherosclerotic lesions ex vivo. A cell-surface protein of approximately 82 kd recognized by this peptide was affinity-purified and determined by mass spectrometry analysis as glucose-regulated protein 78 (Grp78), indicating the surprising presence of this endoplasmic reticulum chaperone on the endothelial cell surface of atherosclerotic lesions. Peptides that mimicked binding functions of their homologues were demonstrated with three peptides homologous to tissue inhibitor of metalloproteinase-2 (TIMP-2), ie, CNHRYMQMC, CNQRHQMSC, and CNNRSDGMC. Phage carrying CNHRYMQMC bound to atherosclerotic lesion endothelium of ApoE knockout mice in vivo. The three peptides bound to endothelial cells in a dose-dependent manner and were inhibited by TIMP-2 protein. These peptides provide a set of probes to interrogate the cell surface repertoire associated with atherogenesis and thrombotic complications.
Figures





References
-
- Libby P: Changing concepts of atherogenesis. J Intern Med 2000, 247:349-358 - PubMed
-
- Faggiotto A, Ross R: Studies of hypercholesterolemia in the nonhuman primate. II. Fatty streak conversion to fibrous plaque. Arteriosclerosis 1984, 4:341-356 - PubMed
-
- Rosenfeld ME, Tsukada T, Gown AM, Ross R: Fatty streak initiation in Watanabe heritable hyperlipemic and comparably hypercholesterolemic fat-fed rabbits. Arteriosclerosis 1987, 7:9-23 - PubMed
-
- Ross R: The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 1993, 362:801-809 - PubMed
-
- Ross R: Atherosclerosis: current understanding of mechanisms and future strategies in therapy. Transplant Proc 1993, 25:2041-2043 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

