Axonal transport of eukaryotic translation elongation factor 1alpha mRNA couples transcription in the nucleus to long-term facilitation at the synapse
- PMID: 14578450
- PMCID: PMC263873
- DOI: 10.1073/pnas.1835674100
Axonal transport of eukaryotic translation elongation factor 1alpha mRNA couples transcription in the nucleus to long-term facilitation at the synapse
Abstract
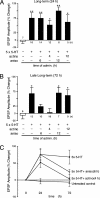
Long-term synaptic plasticity requires both gene expression in the nucleus and local protein synthesis at synapses. The effector proteins that link molecular events in the cell body with local maintenance of synaptic strength are not known. We now show that treatment with serotonin (5-HT) that produces long-term facilitation induces the Aplysia eukaryotic translation elongation factor 1alpha (Ap-eEF1A) as a late gene that might serve this coupling function in sensory neurons. Although the translation factor is induced, it is not transported into axon processes when the stimulation with 5-HT was restricted to the cell body. In contrast, its mRNA is transported when 5-HT was applied to both cell body and synapses. Intracellular injection of antisense oligonucleotides or antibodies that block the induction and expression of Ap-eEF1A do not affect the initial expression of long-term facilitation but do block its maintenance beyond 24 h. The transport of eEF1A protein and its mRNA to nerve terminals suggests that the translation factor plays a role in the local protein synthesis that is essential for maintaining newly formed synapses.
Figures




References
-
- Kandel, E. R. & Schwartz, J. H. (1982) Science 218, 433-443. - PubMed
-
- Kandel, E. R. (2001) Science 294, 1030-1038. - PubMed
-
- Martin, K. C., Barad, M. & Kandel, E. R. (2000) Curr. Opin. Neurobiol. 10, 587-592. - PubMed
-
- Jiang, C. & Schuman, E. M. (2002) Trends Biochem. Sci. 27, 506-513. - PubMed
-
- Kiebler, M. A. & DesGroseillers, L. (2000) Neuron 25, 19-28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

