Dual targeting and function of a protease in mitochondria and chloroplasts
- PMID: 14578924
- PMCID: PMC1326381
- DOI: 10.1038/sj.embor.embor7400011
Dual targeting and function of a protease in mitochondria and chloroplasts
Abstract
Here we show, using the green fluorescent protein (GFP) fusion system, that an Arabidopsis thaliana zinc-metalloprotease (AtZn-MP) is targeted to both mitochondria and chloroplasts. A deletion mutant lacking the amino-terminal 28 residues, with translation initiation at the second methionine residue, was imported into chloroplasts only. However, a mutated form of the full-length targeting peptide, in which the second methionine residue is changed to leucine, was imported to both organelles. No GFP fluorescence was detected when a frame-shift mutation was introduced between the first and second ATG codons of the Zn-MP-GFP construct, suggesting no alternative translational initiation. Our results show that the dual targeting of the Zn-MP is due to an ambiguous targeting peptide. Furthermore, we show that the recombinant AtZn-MP degrades mitochondrial and chloroplastic targeting peptides, indicating its function as a signal peptide degrading protease in both mitochondria and chloroplasts.
Figures



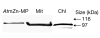

References
-
- Akashi K., Grandjean O. & Small I. ( 1998) Potential dual targeting of an Arabidopsis archaebacterial-like histidyl-tRNA synthetase to mitochondria and chloroplasts. FEBS Lett., 431, 39–44. - PubMed
-
- Bruce B.D., Perry S., Froelich J. & Keegstra K. ( 1994) in Plant Molecular Biology Manual 2nd edn (eds Gelvin, S.B. & Schilferoot, R.A.), J1–J15. Kluwer Academic, Dordrecht, the Netherlands.
-
- Danpure C.J. ( 1995) How can the products of a single gene be localized in more than one intracellular compartment? Trends Cell Biol., 5, 230–238. - PubMed
-
- Duby G., Oufattole M. & Boutry M. ( 2001) Hydrophobic residues within the predicted N-terminal amphiphilic α-helix of a plant mitochondrial targeting presequence play a major role in in vivo import. Plant J., 27, 539–549. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

